Thermal Stability Analysis
Online Inquiry
Analyzing the thermal stability of antibody drugs is one of the most important elements in their quality control. To understand the thermal stability of antibody drugs to ensure their efficacy and safety, Creative Proteomics offers proven methods and advanced technologies. Our comprehensive characterization services help developers accelerate their antibody drug development programs and obtain the most stable potential drug candidates.
Introduction of Thermal Stability Analysis for Antibody Drugs
Thermal stability analysis is an important aspect of antibody drug development and quality control, assessing the ability of a drug to resist irreversible changes in its chemical or physical structure and maintain biological activity at higher temperatures. Temperature changes affect key parameters such as antibody potency, immunogenicity and shelf life. The data obtained from thermal stability analysis assists in optimizing the formulation and manufacturing process of antibody drugs. It helps in the selection of suitable storage conditions and packaging materials that can ensure the drug's stability throughout its shelf life. Creative Proteomics provides thermal stability analysis services for antibody drugs to help clients deeply characterize the structural features of their drugs and understand potential factors that may affect their storage or efficacy.
Thermal Stability Analysis Techniques
Differential scanning calorimetry (DSC) is a thermodynamic technique, which measures heat capacity as a function of temperature. By analyzing DSC-derived thermograms, parameters such as denaturation temperature (Tm) and enthalpy change can be determined, providing customers with information on thermal transitions occurring within the antibody molecule, such as denaturation, aggregation, etc.
FTIR spectroscopy measures the absorption of infrared radiation by the chemical bonds within a sample. FTIR can detect changes in secondary structure, folding, and conformational changes of the antibody during thermal stress. Through this technique we provide information about the protein backbone, hydrogen bonding, and secondary structure elements (e.g., α-helices, β-sheets) of a drug to assess its stability under different temperature conditions.
CD spectroscopy can be used to monitor conformational changes and assess the thermal stability of antibody drugs, providing information about the secondary structure of proteins as well as folding behavior under thermal stress.
Application of Our Thermal Stability Analysis Service
- Providing thermodynamic data that meets regulatory requirements.
- Revealing whether drugs tend to aggregate, which reduces their effectiveness or leads to adverse reactions.
- Selecting and optimizing lead drug candidates, providing information on optimal storage conditions and potential stability issues that can aid in formulation development.
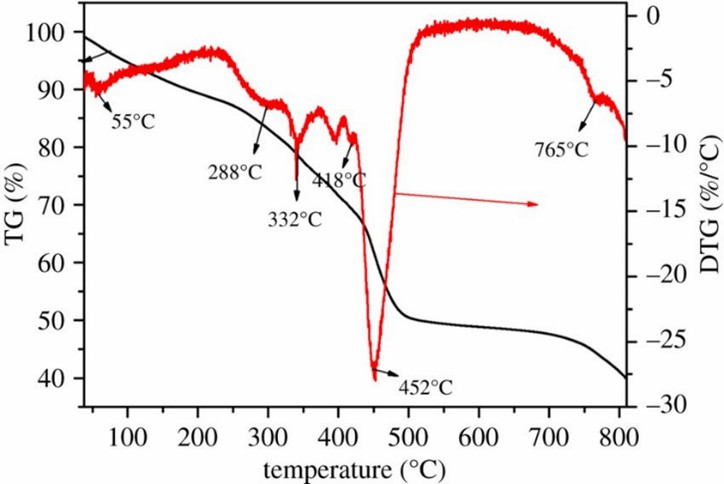 Fig. 1 Thermal stability analysis of WB-g-PAA/LA (5 wt%).
Fig. 1 Thermal stability analysis of WB-g-PAA/LA (5 wt%).
Creative Proteomics' thermal stability analysis service can drive your antibody drug development program forward. We provide thermal stability data to provide insights into your drug's storage, safety, and activity. Contact us to learn more about our thermal stability analysis service of antibody drugs. We will be happy to assist you.

 Fig. 1 Thermal stability analysis of WB-g-PAA/LA (5 wt%).
Fig. 1 Thermal stability analysis of WB-g-PAA/LA (5 wt%).