N-Terminal Modification Analysis
Online Inquiry
Since most recombinant monoclonal antibodies (mAbs) contain glutamic acid or glutamate at their N-terminus, cyclization of these residues to form pyroglutamate is an important degradation pathway that often occurs in therapeutic mAb development. Creative Proteomics offers an advanced N-terminal pyroglutamylation ratio determination service for antibody drugs. Our one-stop testing service can accurately determine the pyroglutamylation rate of antibody drugs, providing useful information for assessing the quality, stability and efficacy of antibody drugs.
What is N-terminal Pyroglutamylation?
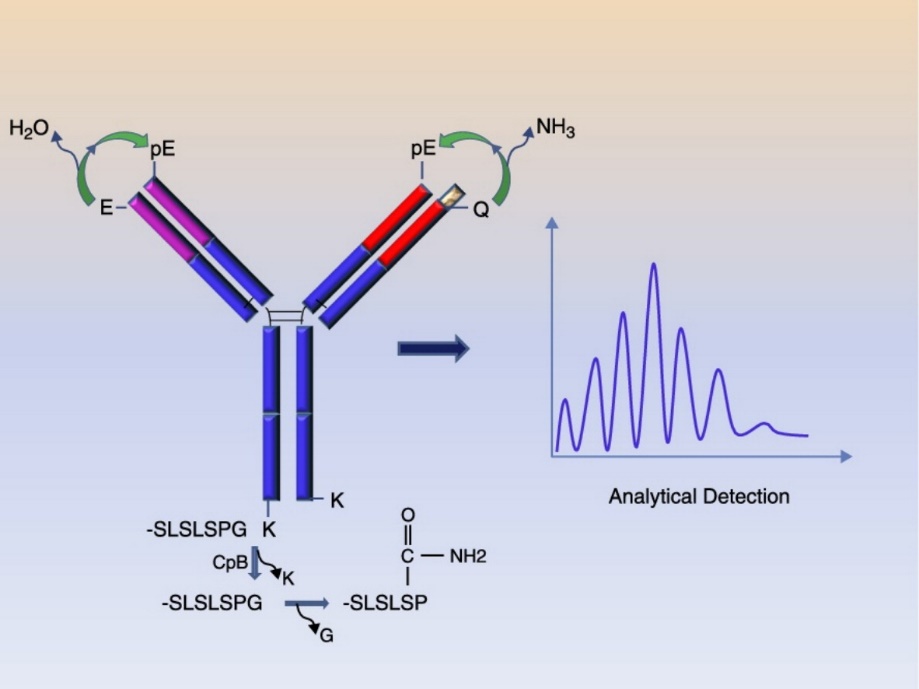
N-terminal pyroglutamylation is a post-translational modification that occurs at the N-terminus of protein or peptide molecules. This modification involves the conversion of N-terminal glutamine or glutamic acid residues into pyroglutamate (pGlu) through the removal of an ammonia group. N-terminal pyroglutamylation is known to impact the stability and biological activity of proteins, including antibody drugs.
Importance of N-terminal Pyroglutamylation Analysis
N-terminal pyroglutaminylation analysis is essential to ensure the quality, efficacy and stability of antibody drug products throughout the manufacturing process and shelf life, and is critical in the development and production of antibody drugs. N-terminal pyroglutamylation rate determination allows assessment of the extent of N-terminal pyroglutamylation, allowing precise adjustment and improvement of manufacturing processes, formulations and storage conditions.
Our N-terminal Pyroglutamylation Ratio Assay Service
At Creative Proteomics, our highly skilled team uses state-of-the-art scientific techniques and equipment to perform N-terminal pyroglutamylation rate assays of antibody drugs. Our services include:
We accept a wide range of antibody drug samples, including different species and isotypes. Our experts conduct a comprehensive analysis to determine the N-terminal pyroglutamylation ratio accurately.
- High precision measurement
We use advanced liquid chromatography-mass spectrometry (LC-MS/MS) based techniques for detection. Liquid chromatography separates peptide fragments based on their physicochemical properties, while mass spectrometry provides information on peptide mass and structure. Ultimately, pyroglutamylated peptides are identified and quantified based on their unique mass and fragmentation patterns.
The data obtained from the LC-MS/MS analysis is processed and analyzed to determine the rate of N-terminal pyroglutamylation. This involves comparing the relative abundance of pyroglutamylated peptides to the total peptide pool.
Services Features of Our N-terminal Pyroglutamylation Ratio Analysis
- High-precision analysis: Ensures accurate measurement of pyroglutamylation levels and ratios using a combination of techniques with high sensitivity and specificity.
- Quantitative assessment: Providing quantitative data on pyroglutamylation levels contributes to the understanding of the dynamics of pyroglutamylation and its potential biological significance.
- Expert interpretation and reporting: Our experienced team of scientists will fully interpret the results and provide a detailed report with graphs, statistical analysis and recommendations for further analysis.
One-Stop Analysis Process at Creative Proteomics
Creative Proteomics provides our clients with authentic, reliable and timely characterization analysis with our advanced technology platform. Contact us to learn more about our N-terminal pyroglutamylation ratio analysis service. We will be happy to assist you.


