Total Antibody Determination
Online Inquiry
Total antibody quantification is considered to be the most useful method to characterize the PK behavior associated with ADC antibodies and to ensure their quality and purity. Creative Proteomics offers a bottom-up LC-MS strategy combined with other assays to ensure accurate characterization of the total antibody content of ADCs.
The Role and Significance of Total Antibody Quantification
Antibody-drug conjugates (ADCs) consist of monoclonal antibodies (mAbs), cytotoxic payloads, and linkers that link the mAbs and cytotoxic payloads. After ADC enters the body, the cytotoxic effector molecules can be gradually dissociated from the main structure by enzymatic or chemical reactions to obtain total antibody (Tab), conjugated antibody (ADC) and free effector molecules (unconjugated drug). Among them, the total antibody is the sum of bound and naked antibodies (DAR=0). As the navigation system of ADC drugs, the antibody portion is mainly responsible for bringing small molecules to the target cells for action, and changes in their concentration in vivo are critical for interpreting the pharmacokinetic characteristics of ADCs. Therefore, accurate quantification of the total antibody content in ADC samples is essential for preclinical pharmacokinetic studies as well as for the successful development of antibody-drug conjugates.
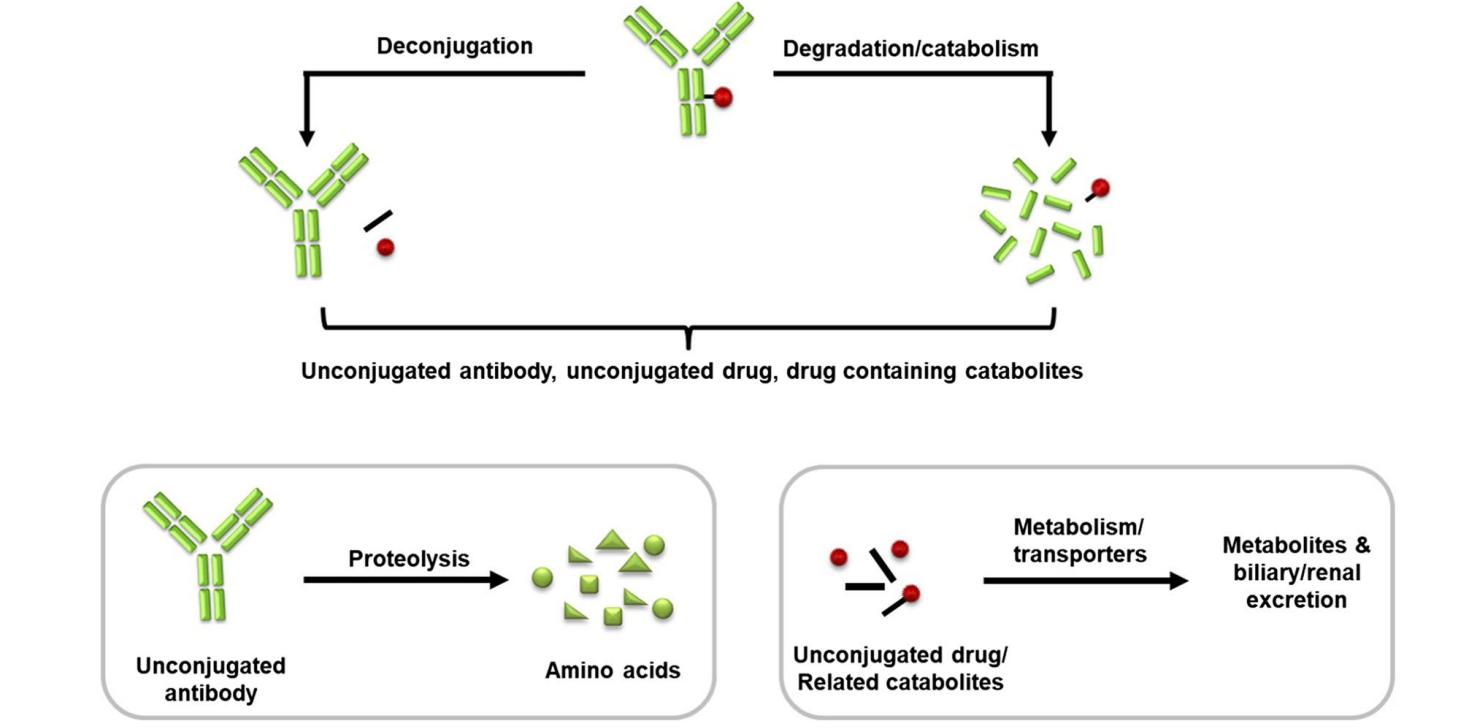 Fig. 1 Conceptualized disposition of an ADC. (Zuo, P., 2020)
Fig. 1 Conceptualized disposition of an ADC. (Zuo, P., 2020)
Total Antibody Quantification Strategy at Creative Proteomics
ADC sample preparation procedures for total antibody content determination are often complex. Our experienced scientists will develop a suitable sample preparation protocol based on the desired target analyte as well as the nature of the sample. During the process, our experts take into account the LC conditions, sample preparation and charge state that may affect the sensitivity of the protein quantification and incorporate affinity capture to accurately quantify the total antibody of the ADC samples to be assayed. Below is our procedure for total antibody quantification:
- The ADCs are extracted from the biomatrices by affinity capture with an anti-idiotypic antibody or generic capture reagents like anti-human IgG against the mAb component of the ADC.
- The affinity-enriched ADC is denatured/reduced, alkylated, and then digested with an enzyme.
- Addition of a stable isotope-labeled internal standard and quantification of a signature peptide selected from complementarity-determining region (CDR) by LC–MS/MS.
Total Antibody Quantification Methods
For total antibodies quantification, Creative Proteomics has a wide range of advanced assay technologies including routine ELISA methods, electrochemiluminescence immunoassays (ECLA) and surface plasmon resonance (SPR). Each of these methods has different characteristics, and our experienced scientists can provide customized assay strategies to accurately quantify total antibody levels in serum, plasma, and tissues, providing valuable insights into the pharmacokinetics and safety assessment of antibody-coupled drugs.
| Methods |
Description |
| ELISA |
- High sensitivity
- High specificity
- Cost-effective
|
| Electrochemiluminescence immunoassays (ECLA) |
- High sensitivity
- Wide dynamic range
|
| Surface plasmon resonance (SPR) |
|
Creative Proteomics is committed to achieving high-precision quantification of total antibodies in a cost-effective manner. To this end, our scientists are developing more innovative alternative methods that allow for highly sensitive quantification of antibodies directly in plasma or tissue homogenates without the need for protein level enrichment. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Zuo, P. Capturing the magic bullet: pharmacokinetic principles and modeling of antibody-drug conjugates. The AAPS Journal. 2020, 22(5): 105.

 Fig. 1 Conceptualized disposition of an ADC. (Zuo, P., 2020)
Fig. 1 Conceptualized disposition of an ADC. (Zuo, P., 2020)