Deamidation Analysis
Online Inquiry
Deamidation is a common post-modification of antibody drugs during production and storage, usually occurring on the side chain of asparagine (Asn, N) and to a lesser extent on glutamine (Gln, Q). Therefore, characterization of deamidation alongside its inter-related modifications, isomerization and racemization, is critically important to understand their roles in antibody druds stability and diseases. Creative Proteomics provides multiscale sample preparation, chromatographic separation and MS techniques for accurate and reliable characterization of antibody protein deamidation in both simple and complex biological samples, yielding important new insight on how deamidation together with isomerization and racemization occurs.
Impact of Deamidation on Antibody Drugs
Deamidation affects the structure and function of antibodies, thereby affecting stability, activity and immunogenicity. First, the negative charge from Glu or Asp residues affects the overall conformation and stability of the protein. This leads to alterations in the antigen-binding region, impairing the ability of the antibody to recognize the target. This in turn affects the binding affinity of the antibody to the target will change. In addition, modified Glu or Asp residues may be recognized by the immune system as foreign antigens, resulting in an immune response in the form of an antibody. This immune response may reduce the effectiveness of the drug by neutralizing or removing antibodies from the body.
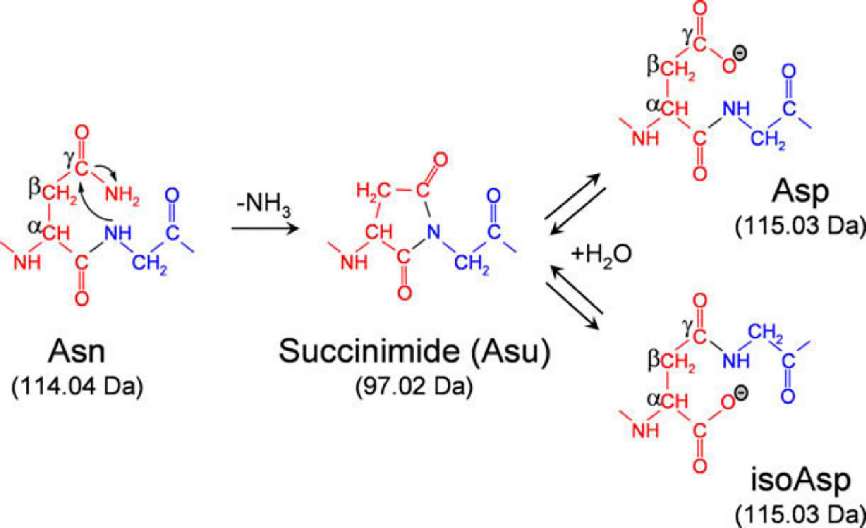 Fig. 1 Mechanism of asparagine deamidation through a succinimide intermediate. (Spanov, B., et al, 2022)
Fig. 1 Mechanism of asparagine deamidation through a succinimide intermediate. (Spanov, B., et al, 2022)
Deamidation Analysis and Characterization Strategies at Creative Proteomics
Creative Proteomics employs a portfolio of advanced detection technologies to comprehensively analyze and characterize deamidation modification sites and deamidation antibody variants in antibody drugs. Our analytical techniques and strategies include the following:
- Exploiting the fact that asparagine deamidation imparts an additional negative charge to the antibody, it is monitored using charge-based assays such as capillary isoelectric focusing (cIEF) or ion exchange chromatography (IEX).
- Confirmation of deamidation sites by bottom-up LC-MS techniques.
- Using the feature that isomerization of aspartic acid to isoaspartic acid leads to more hydrophilic peptides and shorter retention times by RPLC. combining the information provided by RPLC and MS to identify deamidation and aspartic acid isomerization sites.
| Methods |
Principle |
Features |
| IEF |
Isoelectric point |
Fast, sensitive, low specificity |
| 2D-Capillary Electrophoresis |
Isoelectric point |
High sensitivity and good resolution |
| CZE |
Point heterogeneity and Hydrodynamic volume |
Fast and efficient |
| CE-MS |
Charge heterogeneity and quality |
Low sensitivity and poor repeatability |
| RP-HPLC |
Hydrophobicity |
Deep characterization, site-specific and isotopic distribution analysis |
| IEX-HPLC |
Charge heterogeneity |
Simple pre-processing, better resolution, low sensitivity |
| LC-MS |
Molecular weight |
High sensitivity |
What We Deliver?
| Related mass spectrometer parameters |
Mass spectra |
| Raw data |
Deamidation sites and levels in samples |
Features of Our Deamidation Analysis Service
- State-of-art technologies and analytical methods are used to accurately detect and identify deamidation sites of antibody drugs.
- Quantitative analysis of deamidation levels, allowing you to determine the extent of deamidation in your antibody drugs.
- We assess the impact of deamidation on antibody stability, potency, pharmacokinetics, immunogenicity, and other critical factors.
One-Stop Analysis Process at Creative Proteomics

Creative Proteomics provides our clients with authentic, reliable and timely characterization analysis with our advanced technology platform. Contact us to learn more about our deamidation analysis services. We will be happy to assist you.
Reference
- Spanov, B.; et al. Analytical tools for the characterization of deamidation in monoclonal antibodies. Journal of Chromatography Open. 2022, 2: 100025.

 Fig. 1 Mechanism of asparagine deamidation through a succinimide intermediate. (Spanov, B., et al, 2022)
Fig. 1 Mechanism of asparagine deamidation through a succinimide intermediate. (Spanov, B., et al, 2022)