Multi Specific Antibody Drug
Online Inquiry
Multi-specific antibody drugs represent the next generation of antibody-based therapies. Unlike traditional monoclonal antibodies, multispecific antibodies can target two or more disease mechanisms and can offer certain unique therapeutic strategies with more precise targeting and enhanced therapeutic efficacy, which has fueled global enthusiasm for the development of this class of drugs. However, the increased complexity of these antibody therapeutic molecules means that well thought out characterization platforms are required throughout the antibody discovery phase.
At Creative Proteomics, we are dedicated to providing comprehensive multi-specific antibody drug characterization services to our clients worldwide. With our advanced and innovative assay platforms based on mass spectrometry, chromatography and electrophoresis technologies, we are able to provide customized characterization strategies for clients at any stage of drug development. With our expertise in antibody drug development and expanding platform technologies, we can offer a broad portfolio of characterization services to ensure the quality, safety and efficacy of your multi-specific antibody drug.
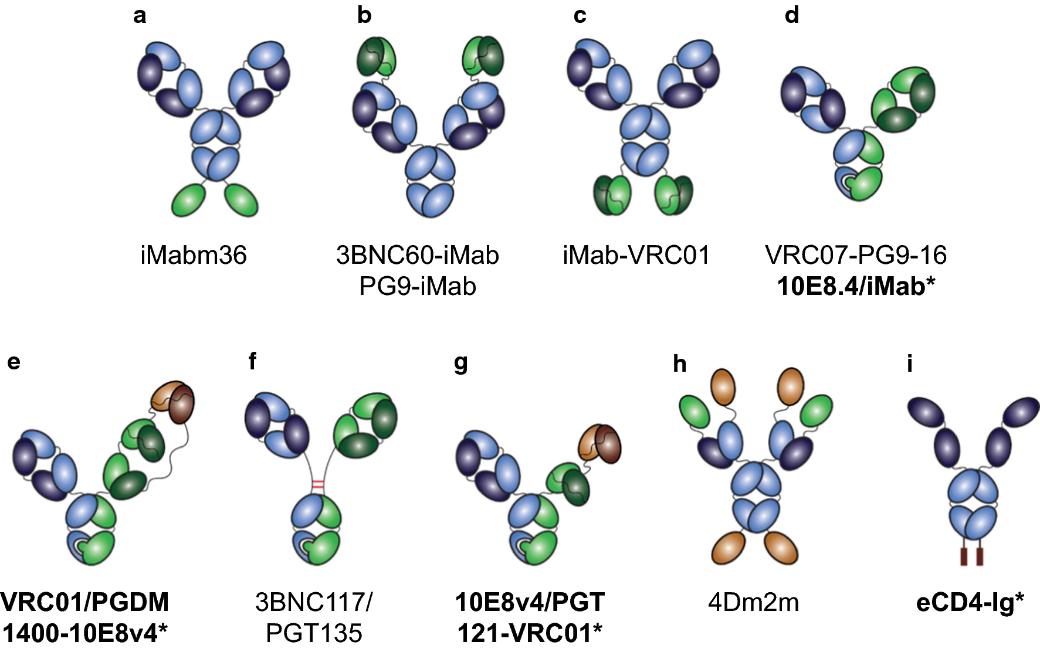 Fig. 1 Engineering multi-specific antibodies against HIV-1. (Padte, N. N. et al., 2018)
Fig. 1 Engineering multi-specific antibodies against HIV-1. (Padte, N. N. et al., 2018)
Multi-Specific Antibody Drug Characterization Services Provided by Creative Proteomics
The key quality attributes of multi-specific antibody drugs encompass several aspects. We can provide the following characterization services for the properties of multi-specific antibody drugs:
Multi-specific antibody drugs usually contain two or more antibody fragments or structural domains, each having the ability to recognize several targets. These fragments can be sourced from immunoglobulin G (IgG) molecules, antibody fragments (such as Fab or scFv), or engineered structural domains (such as nanobodies or structural domain antibodies). Because of their highly complex structure, developing and characterizing multispecific antibody drugs is a daunting task. At Creative Proteomics, we provide a range of analytical techniques to thoroughly analyze the structural and physicochemical properties of multi-specific antibody drugs and ensure proper structural and functional activity. Our expertise allows us to comprehensively characterize these drugs and optimize their effectiveness.
- Molecular weight determination: We use high-resolution mass spectrometry techniques, such as MALDI-TOF, to accurately determine the complete molecular weight of a multi-specific antibody drug and confirm whether there are any missing fragments or mismatches in your antibody molecule.
- Primary structural analysis: We utilize cutting-edge technologies such as peptide map analysis, N-terminal sequencing, and C-terminal sequencing to perform comprehensive structural characterization of antibody drugs. Our comprehensive analytical services can help you confirm whether there are any variations in the protein sequence and termini of your multi-specific antibody drug.
- High-order structure analysis: At Creative Proteomics, we use circular dichroism, nuclear magnetic resonance, mass spectrometry, and many other advanced characterization techniques to help our customers obtain high-order structure-related information about their multi-specific antibody drugs, avoiding quality and safety issues caused by incorrect high-order structures.
- Post-translational modification analysis: With our cutting-edge liquid chromatography and mass spectrometry technologies, we can accurately identify the sites and number of various common post-translational modifications. These modifications typically include disulfide bond modifications, oxidation, deamidation, phosphorylation, glycan modification, glycation modification, N-terminal pyroglutamylation ratio, C-terminal lys deletion, and more.
- Aggregation analysis: Through techniques such as size exclusion chromatography (SEC), dynamic light scattering (DLS), and analytical ultracentrifugation (AUC), we assess the propensity of your multi-specific antibody drug to form aggregates. In addition, we can qualitatively and quantitatively analyze the aggregates formed in your antibody drug. This provides insight into their stability and potential immunogenicity.
- Charge heterogeneity analysis: We use techniques such as capillary isoelectric focusing (cIEF) and capillary gel electrophoresis (CGE) to detect and quantify charge variants, ensuring the consistency and purity of your drug product.
Multi-specific antibody drugs are uniquely characterized in terms of their biological activity, including their multi-targeting and high targeting precision in the treatment of complex diseases. We provide customized characterization strategies for multi-specific antibody drugs for comprehensive evaluation of their biological activities.
Binding activity test: Through our surface plasmon resonance (SPR) and bio-layer interferometry (BLI) techniques, we can comprehensively characterize the binding affinity and kinetics of multis-specific antibody drugs to their target molecules.
Effector function test: Our experts perform a comprehensive assessment of the functional activity, potency and specificity of multi-specific antibody drugs through a variety of cell-based and biochemical assays. These assays primarily include ADCC activity test, CDC activity test, ADCP activity test and TDCC activity test.
In vitro pharmacological tests: By providing a series of advanced in vitro antibody drug activity assays, we help our clients to perform in vitro pharmacodynamic screening of antibody drugs and obtain high quality active drug molecules.
We provide comprehensive analysis of the absorption, distribution, metabolism and excretion parameters of multi-specific antibody drugs through in vivo and in vitro assays. The data obtained are essential for assessing the safety, efficacy and potential for drug-drug interactions.
Purity and impurity analysis
With our advanced detection technologies, we ensure the quality and purity of your drugs by accurately identifying and quantifying impurities and contaminants that may be present in the manufacturing processes and products of multi-specific antibody drugs. This may include the analysis and detection of residual DNA, host cell proteins, endotoxins, aggregates and other unwanted components.
Creative Proteomics offers a range of stability testing services designed specifically for multi-specific antibody drugs, including thermal stability studies, forced degradation studies, accelerated stability studies, and long-term stability studies. Our comprehensive characterization services provide information on the stability, degradation pathways, and shelf life and storage conditions of multi-specific antibody drugs.
Why Choose Us?
- Customized characterization strategies and optimal analysis techniques for the structural and functional characteristics of multi-specific antibody drugs.
- Advanced characterization techniques and proven antibody drug evaluation systems ensure the accuracy and reliability of results.
- Timely delivery of comprehensive analytical reports with expert interpretation and recommendations within the agreed time frame to optimize the development of your multi-specific antibody drugs.
- Our characterization services comply with current regulatory guidelines and industry best practices and provide comprehensive documentation and support to facilitate regulatory submissions.
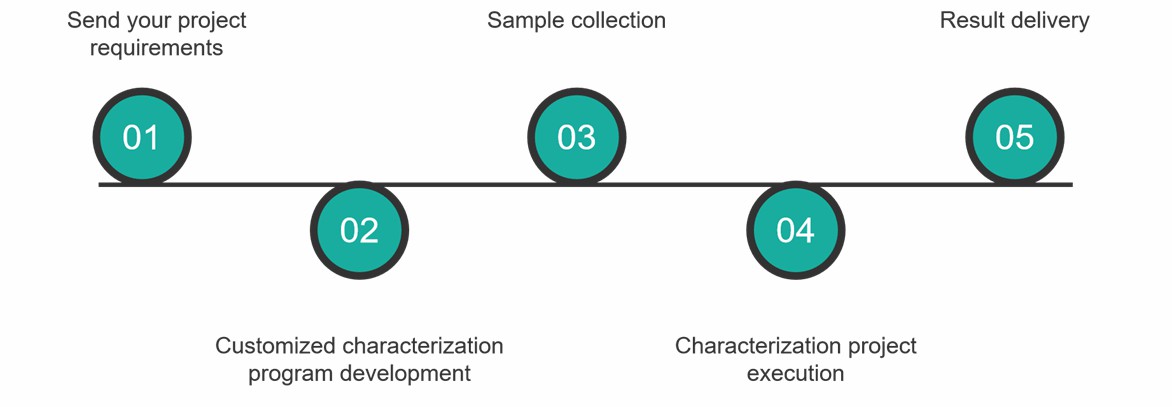
Characterization Service Process
At Creative Proteomics, you can get comprehensive multi-specific antibody drug characterization services by following these simple steps.
With extensive experience and technical expertise in antibody drug characterization, Creative Proteomics is your trusted partner of choice. Our comprehensive services, scientific expertise, and commitment to quality ensure that your drug development journey is supported by accurate and reliable analytical data. Contact us to learn more about how our characterization services can help you achieve your multi-specific antibody drug development goals.
Reference
- Padte, N. N.; et al. Engineering multi-specific antibodies against HIV-1. Retrovirology. 2018, 15(1): 1-17.

 Fig. 1 Engineering multi-specific antibodies against HIV-1. (Padte, N. N. et al., 2018)
Fig. 1 Engineering multi-specific antibodies against HIV-1. (Padte, N. N. et al., 2018)