Hydrophobic Interaction Chromatography (HIC)
Online Inquiry
Hydrophobic interaction chromatography (HIC), has long been used as the gold standard to characterize the protein size, charge and structural variants arising from protein aggregation, clipping and formation of PTMs. In particular, among the several liquid chromatographic modes applied for the characterization of mAbs and ADCs, HIC is the preferred technique for determining the relative hydrophobicity of mAbs and to separate the different populations of ADC molecules. Creative Proteomics enables customers to gain a deeper understanding of their antibody drugs by integrating high-resolution HIC technology into our comprehensive suite of characterization techniques. This includes assessing their purity, aggregation state, aggregation propensity, variant analysis and binding affinity to determine the quality and purity of the antibody drug to optimize drug development and process quality control.
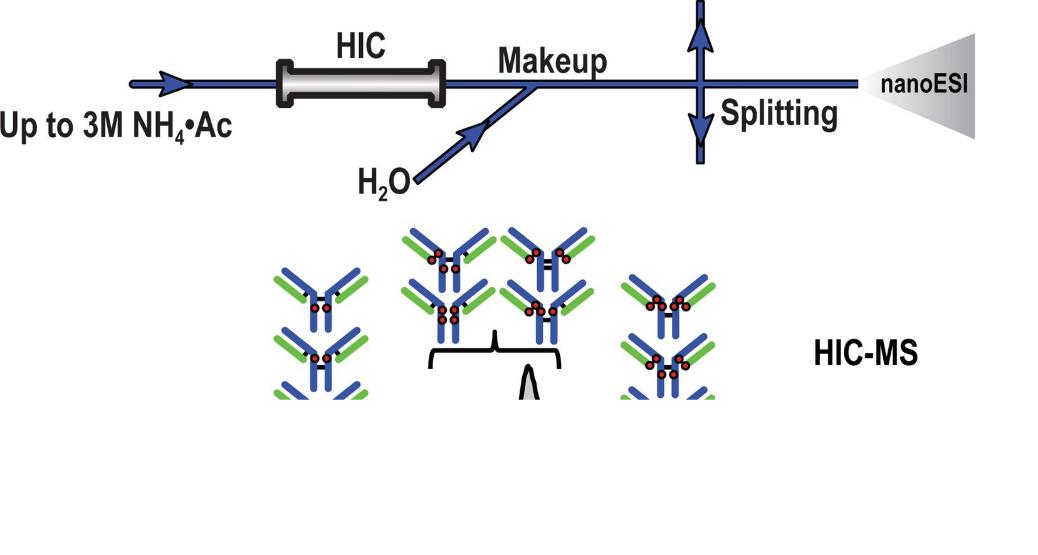 Fig. 1 Successful online coupling of analytical HIC to native MS for the characterization of mAbs and mAb-related products. (Yan, Y. et al., 2020)
Fig. 1 Successful online coupling of analytical HIC to native MS for the characterization of mAbs and mAb-related products. (Yan, Y. et al., 2020)
Application of HIC in Characterization of Antibody Drugs
Hydrophobic interaction chromatography (HIC) is a high-resolution technique. HIC and other orthogonal methods utilizing changes in hydrophobicity are widely used for the characterization and analysis of antibody drugs. Its applications mainly include the following:
- Monitoring of various post-translational modifications.
- Determination of ADCs' average DAR and DAR distribution.
- Determination of mAbs or ADCs hydrophobicity.
- Determination of heterodimerization efficiency of bispecific antibodies.
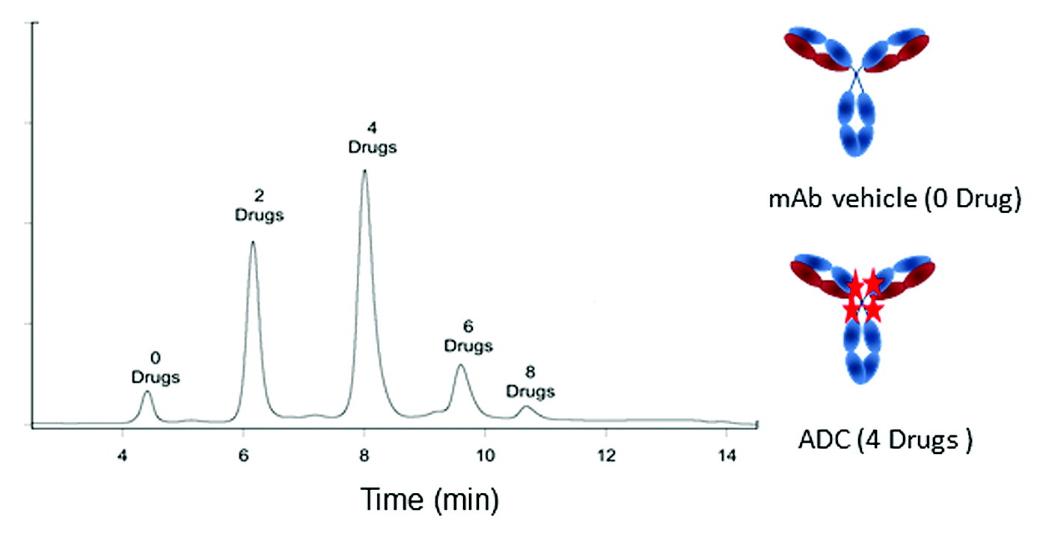 Fig. 2 Antibody to drug ratio determined for an ADC on a TSKgel ether-5PW HIC-HPLC column. (Haverick, M. et al., 2014)
Fig. 2 Antibody to drug ratio determined for an ADC on a TSKgel ether-5PW HIC-HPLC column. (Haverick, M. et al., 2014)
Advantages of Hydrophobic Interaction Chromatography
Key benefits of our HIC technology include:
- HIC is ability to perform separations under non denaturing conditions (i.e. physiological pH conditions, ambient mobile phase temperature and no need for organic solvents), which is particularly useful for ADCs characterization.
- HIC allows for the separation and purification of antibody drugs based on subtle differences in their hydrophobic properties, resulting in enhanced resolution and selectivity.
- Compatibility with a wide range of solvents and buffer systems provides flexibility for characterization and method development for different types of antibody drugs.
Our Capabilities
- State-of-the-art HIC technology
Creative Proteomics has advanced HIC technology that combines high resolution, excellent reproducibility, and high dynamic binding capacity. Our experts have built a comprehensive chromatography platform and equipped it with commonly used HIC columns and chromatography systems, including column chemistries with different separation efficiencies, such as C4, C8, and C18. Using our advanced HIC technology, we can meet the challenges of characterizing different antibody drugs, separating molecules efficiently and accurately to ensure accurate analysis and characterization of antibody drugs.
- HIC method development and validation
Method development and validation are critical stages in the characterization of antibody drugs to obtain reliable and robust analytical tests. Our team of experts works closely with our clients to understand their specific demands and develop efficient and selective HIC characterization methods that ensure accurate identification and quantification of key quality attributes of antibody drugs. In addition, we perform rigorous validation tests to assess the performance and reliability of the HIC methods we offer, giving our clients confidence in the data we generate.
- Key parameters optimization
Creative Proteomics has accumulated extensive experience and expertise in antibody drug characterization using HIC technology. We have a clear understanding of the important parameters that affect HIC selectivity and retention, and are committed to providing our customers with optimized analytical conditions to ensure accurate characterization results. Our scientists perform parameter optimization during the development of HIC-based drug assays, including the concentration and nature of salt, pH of the mobile phase, percentage of organic modifier, gradient shape, and flow rate.
Creative Proteomics is committed to providing HIC-based analytical support throughout the drug development process for optimized methods and data support. Contact us to learn more about our service and we will be happy to serve you.
References
- Haverick, M.; et al. Separation of mAbs molecular variants by analytical hydrophobic interaction chromatography HPLC: overview and applications. MAbs. 2014, 6(4): 852-858.
- Yan, Y.; et al. Online coupling of analytical hydrophobic interaction chromatography with native mass spectrometry for the characterization of monoclonal antibodies and related products. Journal of Pharmaceutical and Biomedical Analysis. 2020, 186: 113313.
- Fekete, S.; et al. Hydrophobic interaction chromatography for the characterization of monoclonal antibodies and related products. Journal of pharmaceutical and biomedical analysis. 2016, 130: 3-18.

 Fig. 1 Successful online coupling of analytical HIC to native MS for the characterization of mAbs and mAb-related products. (Yan, Y. et al., 2020)
Fig. 1 Successful online coupling of analytical HIC to native MS for the characterization of mAbs and mAb-related products. (Yan, Y. et al., 2020) Fig. 2 Antibody to drug ratio determined for an ADC on a TSKgel ether-5PW HIC-HPLC column. (Haverick, M. et al., 2014)
Fig. 2 Antibody to drug ratio determined for an ADC on a TSKgel ether-5PW HIC-HPLC column. (Haverick, M. et al., 2014)