Fab Like Antibody Fragment
Online Inquiry
Fab fragment antibodies retain intact antigen-binding ability and are well characterized for therapeutic applications. Compared to intact antibodies, Fab shows a greater ability to penetrate dense tissues (e.g. solid tumors), thus playing a great role in clinical diagnosis and therapy. At Creative Proteomics, we offer characterization services specifically for Fab-based drugs, providing our customers with information on many aspects of Fab fragment antibody structure, activity and stability to support the development of this class of drugs.
Introduction of Fab Fragment Antibody
Fab fragments, also called antigen-binding fragments, are regions of the antibody structure that can bind to antigen. It consists of a complete light chain (variable and constant regions) and a partial heavy chain structure (variable region and a constant region fragment), which is connected to the heavy chain by a disulfide bond, and is small in size with a molecular weight of 47-48 kDa. Fab class antibody fragments have the characteristics of small molecular mass, high specificity of tissue distribution, low immune prototype, and can be genetically engineered, etc., which make Fab become one of the important members in the field of pharmaceutical research. Fab class drugs have a wide range of applications in the fields of prevention, diagnosis, and treatment. For example, certolizumab ester (a Fab fragment), which is already on the market, can effectively target rheumatoid arthritis, and metuximab I, which is a Fab fragment, has also been used in the treatment of lung cancer.
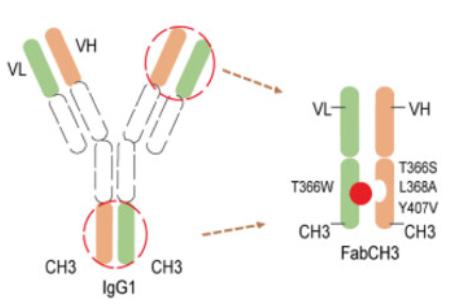 Fig. 1 Fab-Like Antibody Fragment, FabCH3. (Wang, C. et al., 2022)
Fig. 1 Fab-Like Antibody Fragment, FabCH3. (Wang, C. et al., 2022)
Fab-Like Antibody Fragment Characterization Services at Creative Proteomics
Creative Proteomics has built a one-stop antibody drug characterization service platform that provides comprehensive characterization services and accurate and reliable analytical data for the development, optimization, and regulatory submission of Fab class antibody drugs.
Here's an introduction to our characterization services:
- Structural and physicochemical characterization: including molecular weight determination, primary structure analysis, higher order structure analysis, post-translational modification analysis, etc.
- Binding specificity and affinity characterization: advanced biophysical techniques are used to comprehensively characterize the binding activity, specificity, and antigen-antibody interactions of Fab antibody fragments to ensure the bioactivity of this class of drugs.
- ADME characterization: combining in vivo and in vitro assays allows us to accurately and comprehensively analyze and characterize the potential immunogenicity of fab fragment antibody analogs to ensure their safety.
- Stability assessment: with our advanced detection technologies, we can analyze the aggregation tendency and activity of Fab fragment antibodies under various conditions to assess their stability. Our analyses provide insights into drug storage and formulation optimization.
- Batch-to-batch consistency assessment: we offer comprehensive quality control testing to ensure lot-to-lot consistency, which includes purity testing, impurity analysis, size and charge variants, and more.
Characterization Service Process
At Creative Proteomics, you can get comprehensive Fab-like antibody fragment characterization services by following these simple steps.
Creative Proteomics has extensive experience in antibody drugs characterization, we understand the unique requirements of the field and are committed to supporting your Fab-like antibody drug development process with our comprehensive analytical services. Our state-of-the-art technologies and extensive expertise ensure that we support our clients with accurate and reliable data in a timely manner. Contact us for more information and we will be happy to assist you.
Reference
- Wang, C.; et al. Design of a Novel Fab‐Like Antibody Fragment with Enhanced Stability and Affinity for Clinical use. Small Methods. 2022, 6(2): 2100966.

 Fig. 1 Fab-Like Antibody Fragment, FabCH3. (Wang, C. et al., 2022)
Fig. 1 Fab-Like Antibody Fragment, FabCH3. (Wang, C. et al., 2022)