Capillary Zone Electrophoresis (CZE)
Online Inquiry
Capillary zone electrophoresis (CZE) is the most direct and popular electrophoretic mode and is one of the most commonly used tools for the separation and characterization of monoclonal antibody (mAb) charge variants. Creative Proteomics uses CZE technology for antibody drug characterization to determine drug purity, structural integrity and charge heterogeneity. The technology provides a rapid, cost-effective analytical method to help researchers assess the quality, consistency and stability of antibody drugs during drug development. This analysis is critical to ensure the effectiveness and safety of antibody therapies.
Introduction of Capillary Zone Electrophoresis
CZE is known as the most straightforward and popular electrophoretic mode used. The separation is based on the difference of electrophoretic migration of analytes in a background electrolyte (BGE) depending on their charge-to-size ratio. The classical workflow consists to fill up the capillary with BGE. After hydrodynamic or electrokinetic injection of sample, an electrical field is applied allowing the electrophoretic migration of analytes. The most common used detection mode is UV detection mainly fixed at 200 nm or 214 nm for mAbs characterization.
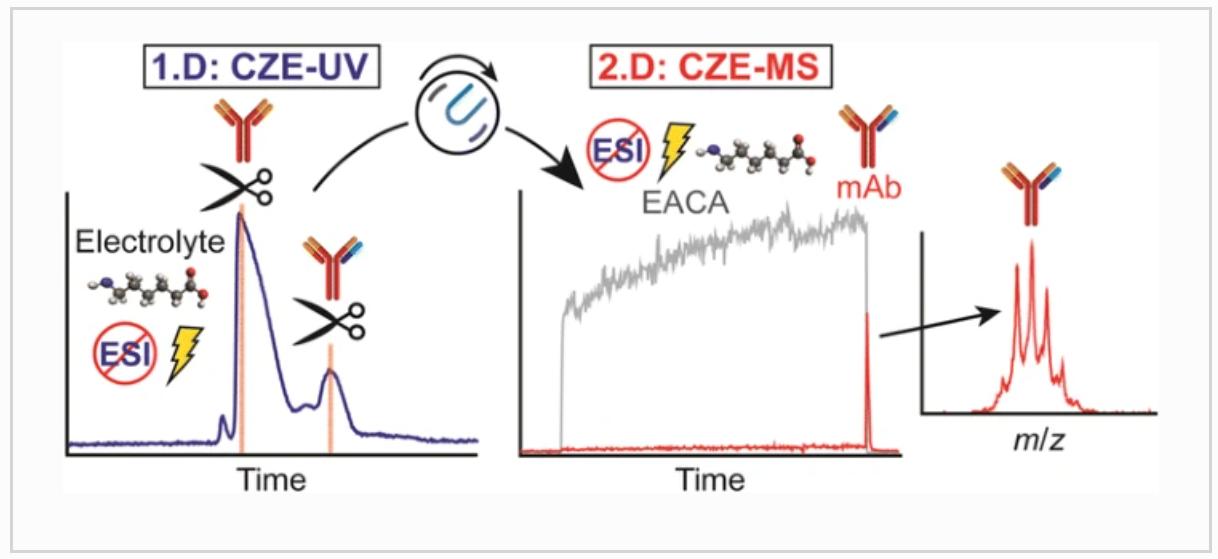 Fig. 1 Two-dimensional capillary zone electrophoresis-mass spectrometry for the characterization of intact monoclonal antibody charge variants. (Jooß, K. et al., 2017)
Fig. 1 Two-dimensional capillary zone electrophoresis-mass spectrometry for the characterization of intact monoclonal antibody charge variants. (Jooß, K. et al., 2017)
CZE is widely used for antibody drug characterization due to its ability to separate and detect different antibody variants and molecular fragments at high resolution. It can provide valuable information about various properties of antibody drugs, including:
- Purity analysis
- Charge heterogeneity analysis
- Size heterogeneity analysis
- Glycosylation analysis of antibodies
- Antigen binding affinity
Advantages of Capillary Zone Electrophoresis
- Better compatibility with MS assays for enhanced analysis of sample structural features.
- CZE offers higher separation resolution and has the advantage of faster separations.
- CE can be used under mild conditions without the need for organic solvents, which is of particular interest for the study of conformers and small non-covalent oligomers.
- CZE for different products is very easy. Sample preparation and separation are fast and allow high throughput applications.
Our Capabilities
- CZE method development and optimization
Our experts have extensive expertise in CZE method development and optimization, providing customized method development services and comprehensive validation testing based on our clients' sample characteristics and assay demands. Our services specifically include:
- Selection of buffer and pH conditions
- Optimization of separation parameters (e.g., voltage, capillary temperature)
- Sample preparation techniques for CZE analysis
- Validation and system suitability testing, including the evaluation of the specificity, linearity, quantitation limit, precision (repeatability and intermediate precision), accuracy, range, and robustness.
- Hyphenation of CE with MS and other complementary technologies
Hyphenation of CE with MS is emerging as a promising bioanalytical tool, and its high separation efficiency and mass spectrometry selectivity are attracting more and more attention in the characterization of antibody drugs. Creative Proteomics, as an expert in the field of antibody characterization, we keep abreast of the development of new technologies in the industry. We offer the integration of CE with mass spectrometry or other complementary technologies to help our customers perform more comprehensive and in-depth analyses of antibody drugs.
- CZE Data presentation and interpretation
We offer expert data interpretation and provide comprehensive analytical reports for in-depth characterization of antibody drugs. Our experience and expertise in data analysis helps our clients make informed decisions during antibody drug development and manufacturing.
Creative Proteomics provides advanced capillary zone electrophoresis (CZE) technology and related characterization services to customers worldwide. Whether you need assistance with CZE method development, data interpretation, or integration with complementary techniques, our team is ready to support you in the characterization of antibody drugs. Contact us to learn more about our service and we will be happy to serve you.
References
- Jooß, K.; et al. Two-dimensional capillary zone electrophoresis-mass spectrometry for the characterization of intact monoclonal antibody charge variants, including deamidation products. Analytical and bioanalytical chemistry. 2017, 409: 6057-6067.
- Gahoual, R.; et al. Cutting-edge capillary electrophoresis characterization of monoclonal antibodies and related products. Journal of Chromatography B. 2016, 1032: 61-78.

 Fig. 1 Two-dimensional capillary zone electrophoresis-mass spectrometry for the characterization of intact monoclonal antibody charge variants. (Jooß, K. et al., 2017)
Fig. 1 Two-dimensional capillary zone electrophoresis-mass spectrometry for the characterization of intact monoclonal antibody charge variants. (Jooß, K. et al., 2017)