Antibody Drug Conjugates Bioanalysis
Online Inquiry
Antibody-drug conjugates (ADCs) are a rapidly growing class of novel biotherapeutic drugs primarily for the treatment of cancer. The ADC molecules are typically composed of cytotoxic small molecule drugs covalently bound to monoclonal antibodies via a linker. Due to the complexity and diversity of antibody-drug couplings (ADCs), comprehensive and informative bioanalytical methods are needed to deepen the understanding of pharmacokinetics (PK) to effectively assess the safety and efficacy of ADCs.
Creative Proteomics has extensive experience in the field of antibody drug characterization. Our characterization experts have established an integrated suite of bioanalytical strategies including both ligand-binding assays and liquid chromatography coupled with mass spectrometry-based assays, typically used for large- and small- molecule bioanalysis, respectively. Our expertise and advanced testing technologies enable us to perform accurate and reliable assessment on a wide range of ADC drug molecules, linker stability, payload distribution and metabolic distribution. Quantitative bioanalytical data from ADCs will facilitate early developability assessments to optimize all ADC components (i.e., antibody, drug, and connector) and help ADCs move into late-stage clinical development and the marketplace.
Challenges in the Bioanalysis of ADCs
Because ADCs have the molecular characteristics of both small and large molecule therapeutic drugs, bioanalytical methods typically used for either small or large molecules are necessary. There are a number of different components present in ADCs, and these molecules differ in DAR values and/or in the sites where the antibody binds to the drug. In addition, ADCs made with current manufacturing processes usually contain non-ADC forms, including naked antibodies as well as free small-molecule drugs. And when the ADC molecules enter the body, the coupled drugs are released from the ADCs over time during circulation, leading to changes in the DAR, and their efficacy and safety may also be affected. For the different types of analytes mentioned above, it is necessary to choose different analytical means to complete the detection according to the different purposes of drug development and evaluation as well as the characteristics of the substances to be tested, which makes the bioanalysis of ADCs very challenging.
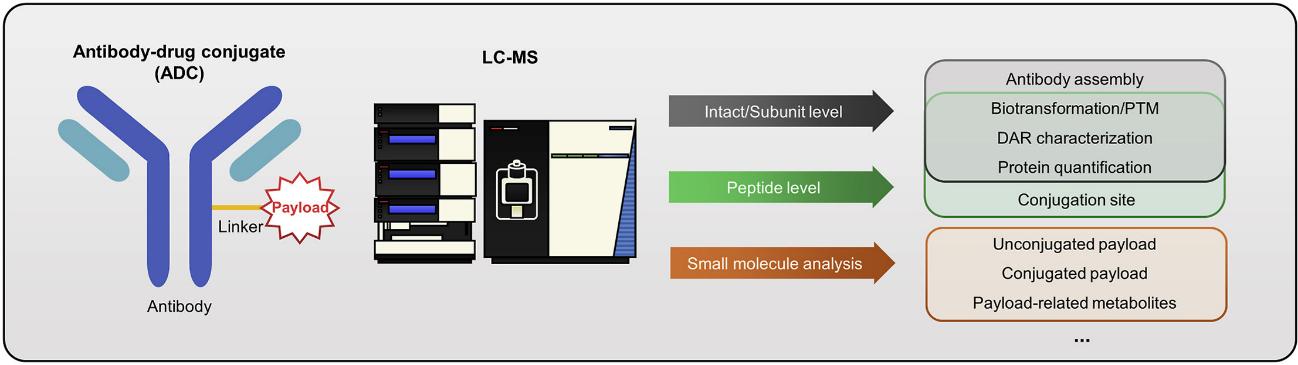
Our Services
ADCs typically incorporate both large and small molecule characteristics. Therefore, a wide range of bioanalytical methods are required to fully characterize them, including physicochemical and pharmacokinetic properties. Creative Proteomics has extensive bioanalytical capabilities that leverage our high-precision platform technology to rapidly characterize key ADC properties to accelerate our customers' ADCs development programs. Below are the assay options we offer for ADC bioanalysis, as well as commonly used bioanalytical methods:
Bioanalytical Platforms at Creative Proteomics
Due to the biotransformation of ADCs, different in vivo forms can be produced, which brings complexity and challenges to the in vivo bioanalysis of ADCs. Creative Proteomics has established a comprehensive bioanalytical platform, including LBA or LC-MS based methods, or a combination of the two methods, which can provide a comprehensive solution for the quantification and analysis of ADC in plasma and serum.
Creative Proteomics has established a state-of-the-art platform based on the ligand-binding assay (LBA), which is typically used in a sandwich assay format and involves the use of capture and detection reagents that bind specifically to the antibody or payload part of the ADC. As a result, our LBA assay platform offers the multiple advantages of high sensitivity, good precision, and high throughput, making it the favored analytical platform of choice to help our customers with their total Ab assays and ADC measurements.
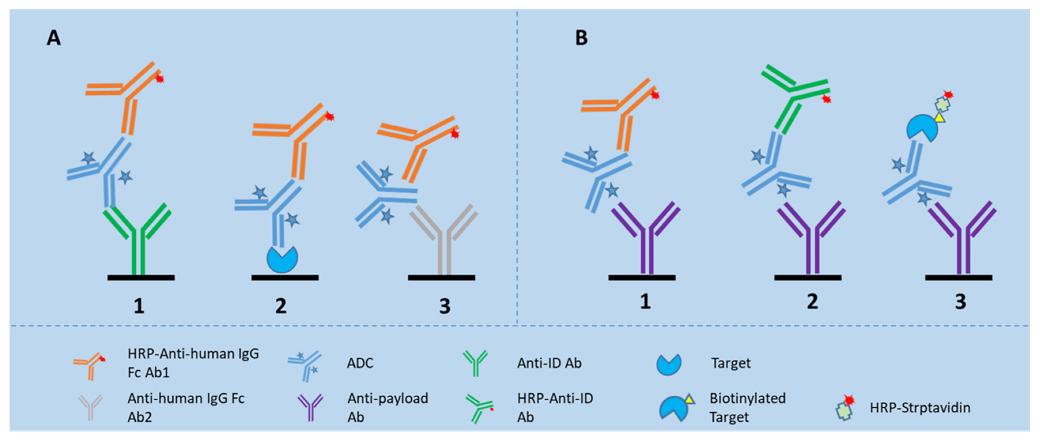 Fig. 1 LBA assay formats for detection of TAb and ADC in the biomatrices. (Qin, Q., et al, 2022)
Fig. 1 LBA assay formats for detection of TAb and ADC in the biomatrices. (Qin, Q., et al, 2022)
Liquid chromatography-mass spectrometry (LC-MS) is a highly versatile and valuable tool that plays a unique role in the quantitative or qualitative analysis of ADCs. Our LC-MS platform contains a variety of highly sensitive detection techniques, including LC-MS/MS, LBA-LC/MS/MS and LBA-LC/HRMS. These methods are highly specific with molecular resolution and are capable of directly determining DAR in ADC samples, thus helping to characterize ADC forms with different DAR values. In addition, our LC-MS-based analytical techniques can be used for total antibody determination, ADC quantification, and small molecule metabolite analysis. Our experienced researchers will select the optimal combination of techniques for each characterization test to provide a comprehensive ADC bioanalytical report.
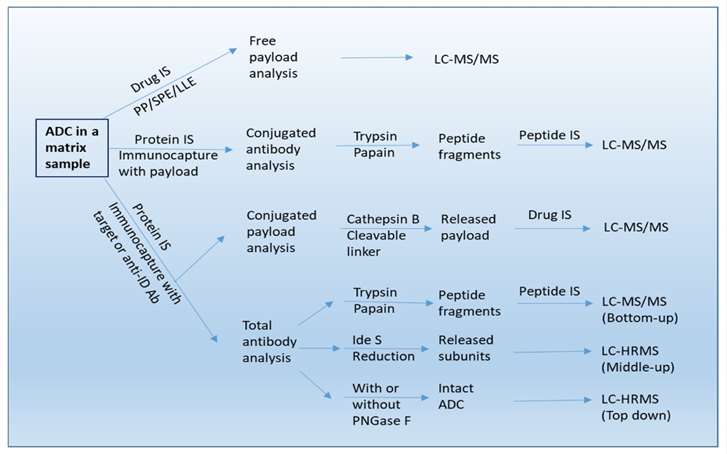 Fig. 2 A workflow of bioanalysis for ADCs in biological sample using LC-MS/MS, LBA-LC/MS/MS, and LBA-LC/HRMS methods. (Qin, Q., et al, 2022)
Fig. 2 A workflow of bioanalysis for ADCs in biological sample using LC-MS/MS, LBA-LC/MS/MS, and LBA-LC/HRMS methods. (Qin, Q., et al, 2022)
Creative Proteomics utilizes our extensive project experience to help clients overcome obstacles in the bioanalysis of antibody-coupled drugs. We work closely with our clients to develop customized analytical solutions to ensure the successful development of safe and effective antibody drug conjugates. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Qin, Q.; et al. Current Analytical Strategies for Antibody–Drug Conjugates in Biomatrices. Molecules. 2022, 27(19): 6299.


 Fig. 1 LBA assay formats for detection of TAb and ADC in the biomatrices. (Qin, Q., et al, 2022)
Fig. 1 LBA assay formats for detection of TAb and ADC in the biomatrices. (Qin, Q., et al, 2022) Fig. 2 A workflow of bioanalysis for ADCs in biological sample using LC-MS/MS, LBA-LC/MS/MS, and LBA-LC/HRMS methods. (Qin, Q., et al, 2022)
Fig. 2 A workflow of bioanalysis for ADCs in biological sample using LC-MS/MS, LBA-LC/MS/MS, and LBA-LC/HRMS methods. (Qin, Q., et al, 2022)