Chromatography and Electrophoresis for Antibody Drug Characterization
Online Inquiry
As more and more antibody drugs are commercially approved for indications expanding beyond oncology, the need for a more comprehensive understanding of this class of therapeutic molecules and control of critical quality attributes (CQA) becomes critical. Separation-based techniques play a key role in CQA characterization and quality control of antibody drugs. Due to the structural complexity and component heterogeneity of antibody drugs, their analysis and quality control face significant technical challenges. Creative Proteomics, as a leading expert in antibody drug characterization, provides chromatographic and electrophoretic analytical tools for high throughput, reliable and rapid methods to our customers in the field of antibody drug development worldwide. With extensive experience in the development, qualification and validation of chromatographic and electrophoretic methods, we provide tailored and cost-effective characterization services to meet the analytical needs of our clients at different stages of antibody drug development.
Application of Chromatography and Electrophoresis to Antibody Drug Characterization
Chromatography and electrophoresis are powerful strategies widely used for antibody drug analysis and characterization. These techniques involve the separation and analysis of complex mixtures of proteins and are essential for ensuring product quality.
Chromatographic techniques, such as size exclusion chromatography and reversed-phase chromatography, are used for separating, purifying, and quantifying antibodies based on their size, hydrophobicity, and charge. They are particularly valuable in monitoring critical quality attributes of antibody drugs, such as purity, aggregation, and degradation. Electrophoretic techniques, such as sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and capillary electrophoresis, are used to analyze the structure, charge, and size of antibodies. These techniques are crucial for assessing protein modifications, such as glycosylation and oxidation, which can affect the efficacy and safety of therapeutic antibodies.
The results of these analyses provide insights into important CQAs associated with antibody drug development manufacturing and quality control, and deepen the understanding of in vitro and in vivo bioactivity and pharmacokinetic studies. The development of these analytical techniques will facilitate early exploitable assessments to optimize all antibody therapeutic drug types and help bring next generation antibody drugs into late-stage clinical development and the marketplace.
Our Chromatography and Electrophoresis Analysis Technologies
Creative Proteomics provides efficient and comprehensive antibody drug characterization services using our advanced chromatography and electrophoresis platform technologies. We offer a broad portfolio of technologies to better meet our clients' characterization needs for different antibody drug types and development stages. The relevant analytical techniques cover different detection mechanisms and involve different assays.
Chromatographic Approaches
HPLC is the ultimate separation technique utilized for both small and large molecules. Our wide range of advanced mass spectrometry techniques enables comprehensive characterization of various quality attributes of antibody drugs.
Ion-Exchange Chromatography (IEX): charge variants analysis
Size Exclusion Chromatography (SEC): evaluation of aggregation or size variants
Hydrophobic Interaction Chromatography (HIC): determination of the relative hydrophobicity of proteins
Reversed Phase Liquid Chromatography (RPLC): molecular weight and peptide map assay
Hydrophilic Interaction Liquid Chromatography (HILIC): N-glycans analysis
Electrophoretic Approaches
Capillary electrophoresis (CE) is a potent technique to analyze biomolecules. Different electrophoretic modes can be used, like capillary gel electrophoresis (CGE), cIEF, icIEF, and capillary zone electrophoresis (CZE). Our electrophoretic-based analytical services provide various opportunities to characterize different antibody drugs and related products, like intact or reduced charge or size variants, isoforms, glycan analyses, PTM, and impurity studies.
Capillary Zone Electrophoresis (CZE):detect impurities detection, charge heterogeneity characterization, glycosylation analysis.
Capillary Gel Electrophoresis (CGE):purity determination, size heterogeneity analysis, charge heterogeneity analysis, drug-antibody ratio determination, stability characterization
Capillary Isoelectric Focusing (cIEF):charge heterogeneity characterization, batch-to-batch consistency, identity verification, stability studies
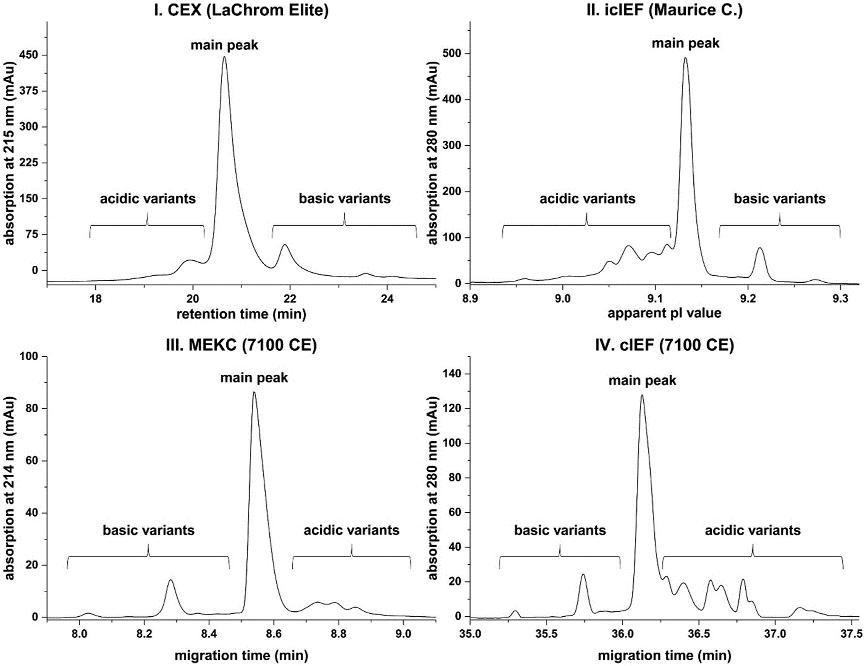 Fig. 2 iCIEF, CZE (MEKC), and IEX (Cation exchange chromatography, CEX) profiles of the NIST mAb standard. (Kaur, H. et al., 2021)
Fig. 2 iCIEF, CZE (MEKC), and IEX (Cation exchange chromatography, CEX) profiles of the NIST mAb standard. (Kaur, H. et al., 2021)
Our Capabilities
- Development and validation of chromatographic and electrophoretic methods for antibody drug characterization.
- Characterization of quality attributes of antibody drugs using our advanced chromatographic and electrophoretic techniques.
- Expert data interpretation detailing the generation of comprehensive reports containing all relevant analytical information.
- Detailed recommendations and insights for the client's antibody drug development program based on the results of the analysis.
Creative Proteomics has extensive experience in the characterization of antibody drugs using chromatographic and electrophoretic techniques. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Kaur, H.; et al. Capillary electrophoresis and the biopharmaceutical industry: Therapeutic protein analysis and characterization. TrAC Trends in Analytical Chemistry. 2021, 144: 116407.


 Fig. 2 iCIEF, CZE (MEKC), and IEX (Cation exchange chromatography, CEX) profiles of the NIST mAb standard. (Kaur, H. et al., 2021)
Fig. 2 iCIEF, CZE (MEKC), and IEX (Cation exchange chromatography, CEX) profiles of the NIST mAb standard. (Kaur, H. et al., 2021)