According to the ICH Q6B guidelines, antibody drugs need to be characterized to confirm whether there are any variations in the protein sequence and ends. In addition, a number of N-terminal changes are frequently observed in drug proteins, including incomplete processing of N-terminal signal sequences, formation of pyroglutamates, and lack of removal of N-terminal methionine residues. Therefore, N-terminal sequencing is an important quality control analysis step for antibody drugs. Creative Proteomics provides professional N-terminal sequencing service to accurately determine the amino acid sequence of the N-terminal end of antibody molecules, helping our customers to better understand the primary structure, stability and functional properties of antibody drugs, providing a powerful tool for antibody drug development.
Importance of N-Terminal Sequencing in The Primary Structural Characterization of Antibody Drugs
For antibody drugs, N-terminal sequencing is particularly important. Because antibodies are complex proteins consisting of two heavy chains and two light chains linked by disulfide bonds, the sequence of the N-terminal (i.e., amino-terminal) of each chain is critical to the antibody's ability to bind specifically. The main roles of this test include the following:
- Confirmation of antibody drug identity: By comparing the N-terminal amino acid sequence to the expected sequence to determine the identity of the looking antibody drug, the possibility of impurities or other contaminants is avoided.
- Evaluation of heterogeneity: N-terminal sequencing is used to identify heterogeneity in the N terminus of antibody drugs due to protein modifications such as signal peptide cleavage, amino acid terminal extension or truncation.
- Monitoring manufacturing processes: Sequence comparison can be used to highlight potential problems or errors that may occur during the production or purification of antibody drugs, helping to optimize and adjust manufacturing processes in a timely manner.
- Stability Assessment: Changes in the N-terminal sequence may affect the stability and degradation properties of antibody drugs.
- Structural analysis: The N-terminal sequence is essential for understanding the overall structure and conformation of the antibody drug.
Antibody Drug N-Terminal Sequencing Platform
Creative Proteomics offers N-terminal sequencing services for antibody drugs based on the Edman sequencing system and high-resolution mass spectrometry. Our advanced technology can help you confirm the complete expression of your antibodies, detect breaks in the expression process, and modify the N-terminal sequence of your antibodies.
Edman degradation method N-terminal sequencing
The Edman sequencing reaction removes the N-terminal amino acids of the antibody one by one through successive chemical reactions, and identifies each removed amino acid. The process can be repeated.
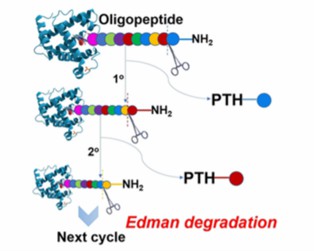 Fig. 1 N-terminal Edman sequencing. (Zhang, et al., 2019)
Fig. 1 N-terminal Edman sequencing. (Zhang, et al., 2019)
1) Allows accurate analysis of N-terminal amino acid sequences, in particular the ability to discriminate between isoleucine and leucine, glutamine and lysine.
2) Directly analyzes amino acid information without any protein database.
Proteins can be submitted as SDS or natural polyacrylamide gel sheets in solution, or blotted onto PVDF membranes.
1) In solution: Samples should be free of interfering buffers, salts and detergents. If interfering buffers, salts are unavoidable, the material may still be sequenced after cleanup. Amines in buffers (e.g. Tris, ethanolamine and glycine) should be avoided.
2) In-gel: Cut off sections of the gel of interest and place in a microcentrifuge tube. Proteins can be eluted from the polyacrylamide. Use Komas Blue R-250 or G-250 instead of silver stain for gel staining whenever possible.
3) On PVDF membranes: SDS-PAGE separated proteins are blotted onto PVDF membranes. Proteins can be stained with Kaumas Blue R-250, Amide Black, Carmine S or Sypro Ruby (silver staining is not recommended).
Mass spectrometry N-terminal sequencing
In MS-based sequencing, antibody proteins are pretreated with digestive enzymes to digest into small fragments, which are then analyzed by mass spectrometry. The collected data is matched against a theoretical sequence database, which in turn confirms the N-terminal sequence.
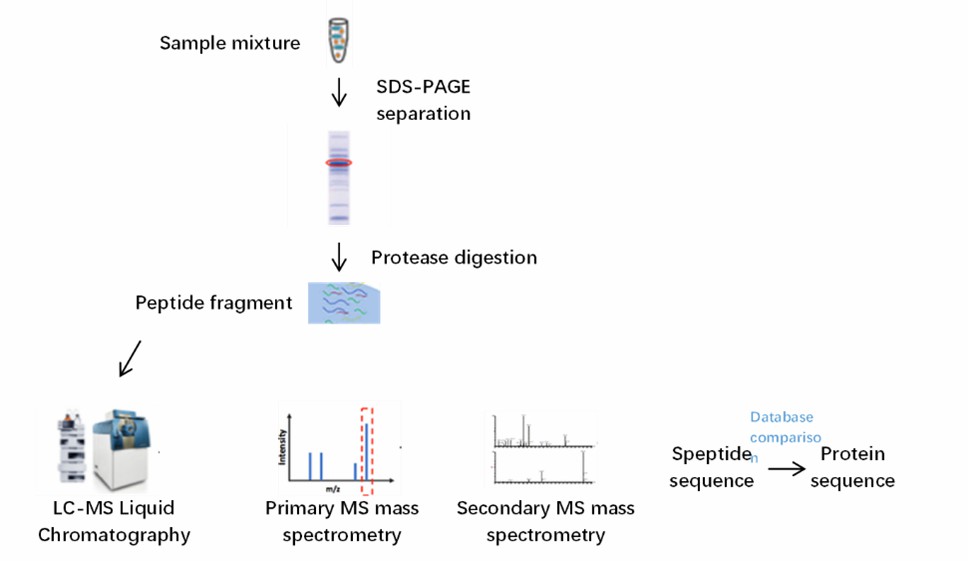 Fig. 2 Mass spectrometry sequencing flow chart.
Fig. 2 Mass spectrometry sequencing flow chart.
1) Mass spectrometry can measure the closed and modified antibody protein drug N-terminal.
2) Preferred full-length antibody sequencing method, complementary to Edman sequencing.
Both gel and liquid protein samples are acceptable, and sample purity should be as high as possible. Mass spectrometry is a very sensitive technique, so samples must be carefully prepared. Factors that may contaminate samples, such as incomplete subcellular separation or purification due to multi-protein complexes, samples overwhelmed by highly abundant proteins, and contamination from skin or hair. Any source that may introduce contaminating proteins should be eliminated.
N-terminal sequencing technology process at Creative Proteomics
N-terminal sequencing is performed to compare and validate the N-terminal amino acid sequence inferred from the gene sequence with the N-terminal amino acid sequence of the antibody drug product. The analytical technique used was the Edelman method or mass spectrometry. This technique determines the amino acid sequence by sequentially cutting amino acids from the N terminus of a protein and obtains a very reliable amino acid sequence. The specific test procedure includes the following:
The antibody drug samples are denatured and reduced to release the N-terminal amino acid residues.
- Separation and purification
N-terminal amino acids are separated by mass spectrometry or electrophoresis techniques.
- Analysis and identification
The isolated N-terminal amino acids are then identified and sequenced by sequencing methods such as automated Edelman degradation or mass spectrometry to determine the amino acid sequence of the sample.
The acquired data is processed, analyzed, and compared to known antibody sequences or proprietary databases to confirm the identity and integrity of the N-terminal sequences.
As a professional antibody drug characterization service provider, Creative Proteomics can provide our clients with N-terminal sequencing service based on Edman sequencing system or mass spectrometry technology. We provide you with comprehensive data analysis reports to help you confirm whether your antibody protein drugs are fully expressed or otherwise mutated. Contact us to learn more about N-terminal sequencing service for antibody drugs. We will be happy to assist you.
Reference
- Zhang, H.; et al. Sensor Array Based Determination of Edman Degradated Amino Acids Using Poly(p-phenyleneethynylene)s. Chemistry. 2020 Jun 23;26(35):7779-7782.

 Fig. 1 N-terminal Edman sequencing. (Zhang, et al., 2019)
Fig. 1 N-terminal Edman sequencing. (Zhang, et al., 2019) Fig. 2 Mass spectrometry sequencing flow chart.
Fig. 2 Mass spectrometry sequencing flow chart.