In Vitro Toxicological Evaluation of Antibody Drugs
Online Inquiry
The in vitro toxicological evaluation of antibody drugs plays a crucial role in the development and regulatory approval process of these drugs. It is an essential step in addressing the safety concerns associated with these potentially life-saving therapeutic agents. Creative Proteomics understands the importance of this evaluation and offers comprehensive, state-of-the-art in vitro toxicology evaluation services for antibody drugs.

The Role of In Vitro Toxicological Evaluation of Antibody Drugs
The primary goal of in vitro toxicological evaluation is to assess the potential adverse effects of antibody drugs on various target organs and systems before they are tested on animals or humans. This early assessment helps identify any potential safety issues and assists in making informed decisions during the drug development process. In vitro toxicology data also serve as a basis for predicting the potential risks and toxicological profile of the drug in vivo.
Our In Vitro Toxicological Evaluation Services of Antibody Drugs
In the labs of Creative Proteomics, our scientists have developed a series of in vitro toxicology tests designed to identify any adverse effects or potential toxicity that a drug may have on different cell types or organs. This evaluation process consists of a well-defined methodology, advanced technology, and a carefully designed service flow. These evaluations are crucial to ensure the safety and efficacy of the drug before it progresses to clinical trials or is approved for use in patients.
The detailed services we offer for in vitro toxicological evaluation of antibody drugs include:
We expose various cell lines to different concentrations of antibody drugs to determine their effects on cell viability and proliferation, helping our clients identify any potential cell-specific toxicity of their antibody drugs.
We use different tests (e.g., Ames assay or micronucleus test) to assess genotoxicity. These tests assess whether an antibody drug may cause cellular DNA damage or mutations.
Immunotoxicity testing assesses whether an antibody drug may adversely affect the immune system. It involves the detection of various immune parameters, including cytokine release, lymphocyte proliferation and antibody production.
- Organ-specific toxicity testing
We use various cell types such as hepatocytes or cardiomyocytes to detect the potential drug-induced toxicity on specific organs or tissues.
- Metabolism and excretion studies
We offer advanced assays to determine the metabolism and efflux of antibody drugs in vivo and to assess the potential for bioactivation or formation of toxic metabolites.
Our customized experimental designs help analyze the absorption, distribution, metabolism, and excretion (ADME) of antibody drugs to better understand their behavior in the body and to identify potential toxicity issues associated with the drugs.
Services Features of Our In Vitro Toxicological Evaluation
- Comprehensive toxicity assessment
- High-throughput screening
- Customized panel of assays
- Predictive toxicology analysis
- Regulatory compliance
Services Flow of Our In Vitro Toxicological Evaluation
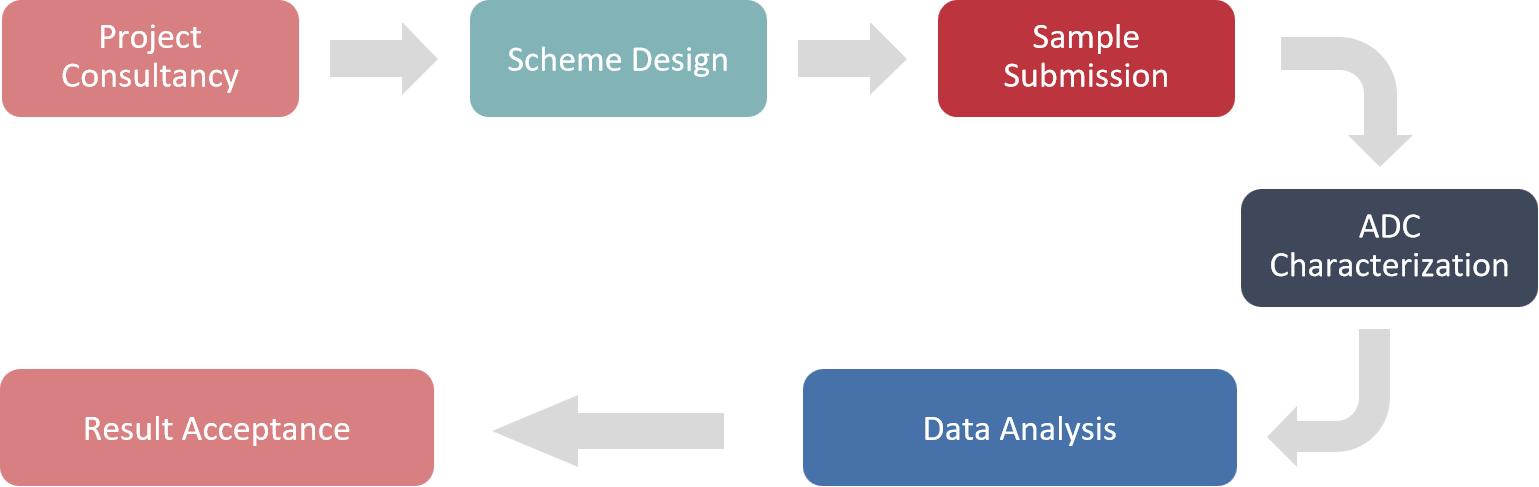
Creative Proteomics has unrivaled expertise in the in vitro toxicological evaluation of antibody drugs and we look forward to working with you. Our dedicated team is ready to support your drug development journey, contributing to the advancement of safer and more effective therapies in the field of antibody drugs. For more information or to request our services, please feel free to contact us.


