nSEC-MS for Antibody Drug Characterization
Online Inquiry
Native size-exclusion chromatography-mass spectrometry (nSEC-MS) is an analytical method that combines SEC with native-MS, a high-resolution mass spectrometry technique. This method enables precise determination of Intact molecular weight and provides an accurate and efficient assay for analyzing antibody drug aggregation, determining the drug/antibody ratio (DAR) of antibody drug conjugates (ADCs), and characterizing post-translational modifications (PTMs). Creative Proteomics possesses a state-of-the-art nSEC-MS analytical platform. Our nSEC-MS technology has high resolution and sensitivity, which streamlines workflow, reduces analysis time, boosts throughput, and facilitates credible and precise antibody drug characterization.
Our nSEC-MS -Based Antibody Drugs Characterization Services
Creative Proteomics' innovative nSEC-MS technology utilizes the separation capabilities of size exclusion chromatography (SEC) and the analytical power of mass spectrometry (MS) to comprehensively characterize antibody drugs. Using advanced nSEC-MS detection tools, we can determine the size, purity, modifications and higher order structure of antibody drugs. We provide professional characterization services and valuable information to our customers at all stages of antibody drug development, including formulation development, stability testing and quality control.
Intact Molecular Weight Determination
Mass spectrometry can accurately measure the molecular weight of eluates separated by SEC to help customers determine the intact molecular weight of an antibody drug product. Our highly sensitive and high-resolution nSEC-MS technology can identify any modifications or alterations, such as truncations, glycosylation changes, or disulfide bond dislocations, to understand the integrity of an antibody drug product.
Post-Translational Modification Analysis
PTMs are one of the key quality attributes of antibody drugs, affecting their biological activity, stability and efficacy. With nSEC-MS technology, we can help our customers accurately identify the presence and level of PTMs such as glycosylation, oxidation and deamidation.
Higher Order Structure Analysis
nSEC-MS can provide information on the higher order structure of antibody drugs. Conformational properties of eluates can be elucidated by ion mobility mass spectrometry (IM-MS). We have used nSEC-MS technology to correlate ion mobility data with mass spectrometry measurements, allowing us to determine the higher order structure of antibody drugs.
Aggregate Analysis
Quantitative analysis of aggregates has received particular attention because aggregates can affect the efficacy and immunogenicity of antibody drugs. Our high-resolution nSEC-MS technology accurately identifies and quantifies the presence of aggregates, including monomers, aggregates and fragments, in antibody drugs.
Drug-Antibody Ratio (DAR) Assay
Our characterization services based on nSEC-MS technology can provide our customers with information on various aspects of DAR determination, including identification and quantification of drug molecules bound to antibodies, mass-to-charge ratios of different species in the sample, distribution of the drug on the antibody molecule, etc. to determine the DAR.
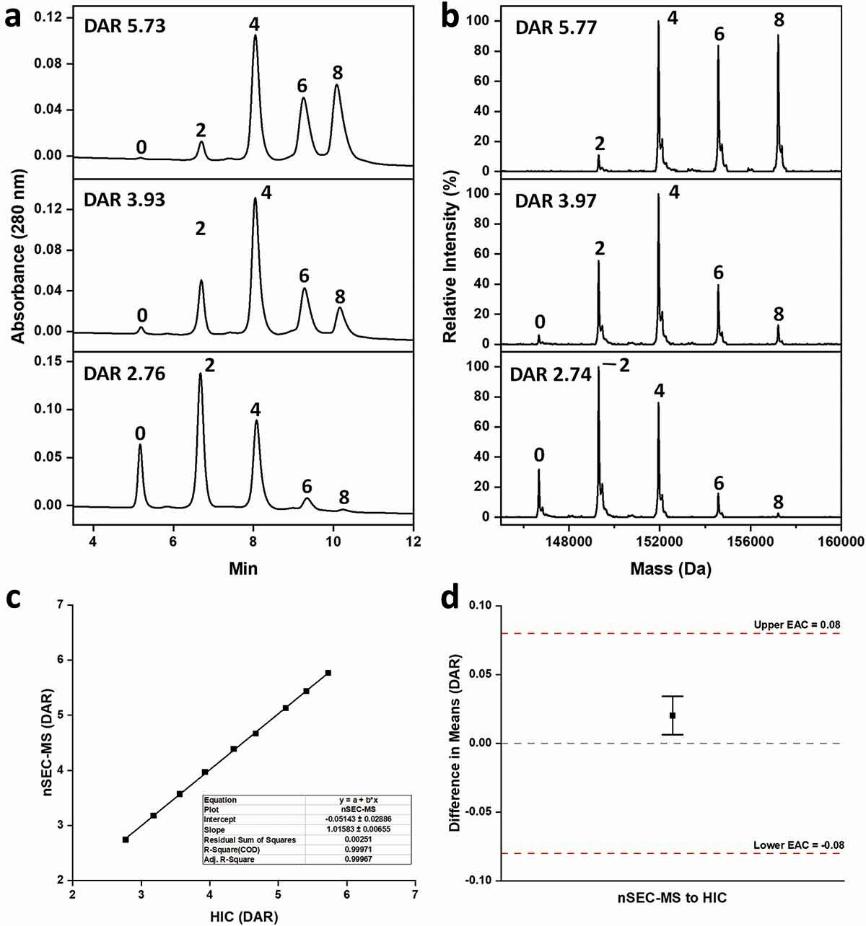 Fig. 1 Comparison of HIC (panel A) and nSEC-MS (panel B) profiles of ADC-A samples at three different DAR levels. (Jones, J. et al., 2020)
Fig. 1 Comparison of HIC (panel A) and nSEC-MS (panel B) profiles of ADC-A samples at three different DAR levels. (Jones, J. et al., 2020)
Advantages of Our nSEC-MS Technology
- The combination of high resolution, high sensitivity and high reproducibility enables reliable and accurate characterization of antibody drugs.
- The extended mass number allows the analysis of larger antibodies, antibody-drug conjugates, and glycoforms.
- High-throughput assays and fast data acquisition significantly decrease the turnaround time for the development of antibody drugs.
- Help clients gain a thorough understanding of the quality and characteristics of antibody drugs by integrating several essential factors into a unified platform.
What We Deliver?
- Experimental steps
- Relevant mass spectrometry parameters
- Mass spectrometry images
- Raw data
- Complete analytical report
Creative Proteomics offers a one-stop assay service based on nSEC-MS technology that provides customers with comprehensive information on the structure, purity and integrity of antibody drugs. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Jones, J.; et al. Native size-exclusion chromatography-mass spectrometry: suitability for antibody–drug conjugate drug-to-antibody ratio quantitation across a range of chemotypes and drug-loading levels[C]//MAbs. Taylor & Francis. 2020, 12(1): 1682895.

 Fig. 1 Comparison of HIC (panel A) and nSEC-MS (panel B) profiles of ADC-A samples at three different DAR levels. (Jones, J. et al., 2020)
Fig. 1 Comparison of HIC (panel A) and nSEC-MS (panel B) profiles of ADC-A samples at three different DAR levels. (Jones, J. et al., 2020)