Exogenous DNA Residue Analysis
Online Inquiry
Exogenous DNA residue analysis plays a crucial role in the development and manufacturing of antibody drugs. Understanding and quantifying the presence of exogenous DNA residues in the final drug products is essential for ensuring their quality, safety, and efficacy. At Creative Proteomics, we offer comprehensive and reliable exogenous DNA residue analysis testing services to pharmaceutical companies and biotechnology firms. With our state-of-the-art facilities and experienced team, we provide accurate and efficient analysis, enabling our clients to meet regulatory requirements and maintain high standards in their antibody drug development.

Importance of Exogenous DNA Residue Analysis of Antibody Drugs
Exogenous DNA refers to DNA that is not naturally present in the organism or cell line used for the production of antibody drugs. This could include bacterial or viral DNA, plasmids, or other foreign genetic material. The presence of exogenous DNA in antibody drugs can have significant implications on both patient safety and regulatory compliance. The detection of exogenous DNA is vital to ensure the purity and integrity of antibody drugs. Even trace amounts of exogenous DNA can potentially cause adverse effects, such as immune responses or contamination with infectious agents. Therefore, regulatory bodies such as the U.S. Food and Drug Administration require thorough testing of antibody drugs for the presence of exogenous DNA. The importance of detecting exogenous DNA in antibody drugs lies in ensuring the safety and efficacy of these therapeutics.
Our Exogenous DNA Residue Analysis
Our exogenous DNA residue analysis testing services encompass a wide range of solutions tailored to meet the specific needs of our clients. We offer qualitative and quantitative analysis of exogenous DNA residues in antibody drug products, excipients, cell banks, and intermediate samples. Our services cover various stages of antibody drug development, including raw material testing, process control, and final product evaluation. Whether you require routine testing or comprehensive analyses for investigational purposes, our dedicated team is ready to assist you.
We employ cutting-edge techniques and advanced methodologies to obtain accurate results in exogenous DNA residue analysis. Our approach involves the use of polymerase chain reaction (PCR) and real-time PCR technologies for the amplification and detection of exogenous DNA residues. By utilizing validated and highly sensitive assays, we can detect and quantify DNA residues at low levels (ppm to sub-ppm). Our methods are in compliance with industry standards, such as ICH guidelines and regulatory requirements.
Our Exogenous DNA Residue Analysis Services Process
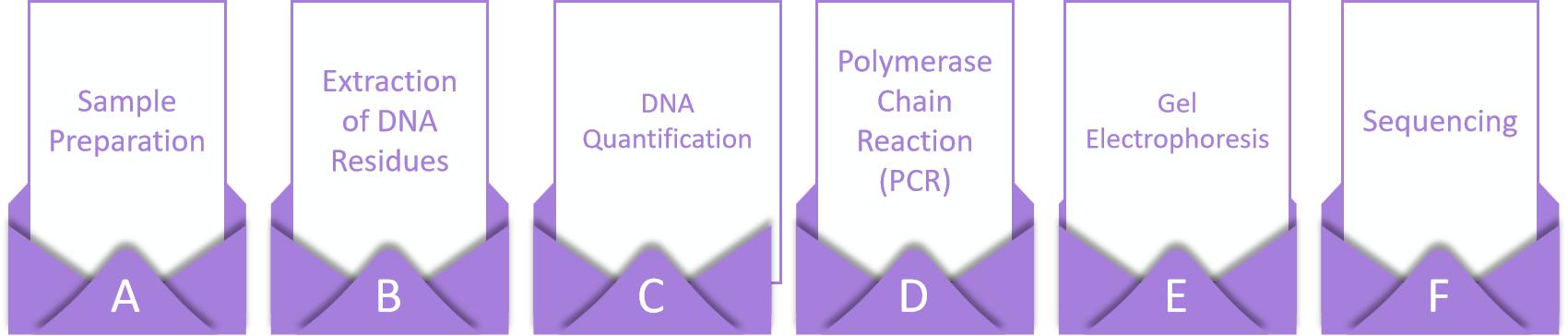
Our Host DNA Residue Analysis process follows a well-defined and quality-driven workflow to ensure reliable and reproducible results. Upon receiving your samples, our laboratory professionals perform a thorough sample preparation procedure, including DNA extraction and purification, DNA quantification, polymerase chain reaction (PCR), gel electrophoresis and sequencing.
Service Features
- Our service covers a wide range of antibody drugs, including monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates.
- Our team works closely with you to understand your specific needs and develop tailored testing solutions that meet your expectations.
- State-of-the-art technology and equipment to ensure the highest level of accuracy and sensitivity in our analysis.
- Strict quality control measures throughout the entire process to ensure reliable and reproducible results.
Creative Proteomics is proud to offer our global customers advanced exogenous DNA residue detection and analysis services for your antibody drug discovery programs. For more information or to request our services, please feel free to contact us.


