Antibody drug conjugates (ADCs) are a new type of therapy that blends the specificity of monoclonal antibody drugs with the potency of small molecule cytotoxic drugs. Due to their targeting abilities and low toxicity in cancer treatment, they have gained immense focus and continuous progress. Nonetheless, ADCs are structurally heterogeneous and extremely intricate, posing challenges for their characterization.
At Creative Proteomics, we specialize in providing comprehensive characterization services to researchers in the field of antibody drug development, providing critical insights for optimizing the development of antibody drug conjugates and accelerating drug launches. Our experienced characterization team can provide customized characterization solutions and analytical methods for different assay needs at all stages of antibody drug conjugates development.
Quality Properties of ADCs
The quality attributes of ADCs include macromolecular attributes, ADC-specific attributes, and small molecule-related attributes.
| Quality Properties of ADCs |
Key Quality Attribute Detection |
| Macromolecular attributes |
- Intact molecular weight
- Peptide maps
- Aggregates
- Charge heterogeneity
- Post-translational modifications
- Detection of macromolecular activity
|
| ADC-specific attributes |
- Total antibody
- Conjugated antibody
- Unconjugated antibody
- Conjugated payload quantitative
- ADCs coupled site
- DAR value
- DAR distribution
|
| Small molecule-related attributes |
- Free drug and related impurities
- Residual reagents
- Heavy metal residues
|
Antibody Drug Conjugates (ADCs) Characterization Services Provided by Creative Proteomics
The analysis and characterization of ADC drugs entail several facets. Our advanced technologies enable us to offer tailored characterization strategies for different aspects of ADCs, such as physicochemical properties, bioactivity, pharmacokinetics, impurities, and stability. This ensures the quality, safety, and stability of this category of antibody therapeutic molecules.
We employ advanced mass spectrometry, liquid chromatography, and electrophoresis techniques to comprehensively determine the physicochemical attributes of ADCs. This encompasses the scrutiny of amino acid sequences in primary structures, the inspection of intact antibodies, the examination of disulfide bonds in higher structures, the analysis of post-translational modifications, evaluation of DAR distribution, purity, aggregation, and charge heterogeneity. Our comprehensive characterization services offer valuable insights that help our customers maintain optimal product quality and streamline their drug development processes. We prioritize clarity and precision in our analysis, ensuring that our assessments leave no room for ambiguity.
Bioactivity is a key factor in ensuring the effectiveness of ADCs. Our characterization of ADCs' bioactivity includes binding affinity and specificity assays for antibody components, cellular activity assays, ADC/CDCC assays, and more. Our advanced surface plasmon resonance (SPR) and enzyme-linked immunosorbent assay (ELISA) assays allow for accurate and comprehensive analysis of the biological activity of ADCs. These results provide critical information for evaluating and optimizing the therapeutic efficacy of ADCs.
Understanding the pharmacokinetic properties of antibody drugs is critical for assessing their therapeutic efficacy and safety. At Creative Proteomics, we employ in vivo and in vitro methods to comprehensively characterize the absorption, distribution, metabolism, and elimination (ADME) of ADCs. Our advanced characterization techniques and expertise allow us to provide valuable data for formulation optimization, dosage selection, and prediction of clinical outcomes for ADCs.
Stability is an important aspect of antibody drug conjugates development. We provide a comprehensive analysis of the stability of ADCs under various stress conditions through accelerated and long-term stability studies. Our studies help identify degradation pathways, assess the impact of formulation and storage conditions, and guide the identification of robust ADCs development programs.
Why Choose Us?
- Our expertise in antibody drug discovery allows us to provide clients with dependable characterization results and expert guidance.
- We utilize advanced mass spectrometry, chromatography, and electrophoresis technologies for optimal accuracy and reliability.
- Our customized solutions cater to the unique ADCs, development stages, and characterization needs of our clients.
- We strictly adhere to regulatory guidelines and implement rigorous quality testing standards to ensure data reliability and compliance.
- Our efficient and streamlined workflows enable us to deliver precise and dependable data within agreed-upon timeframes, while also providing expert interpretation and advice.
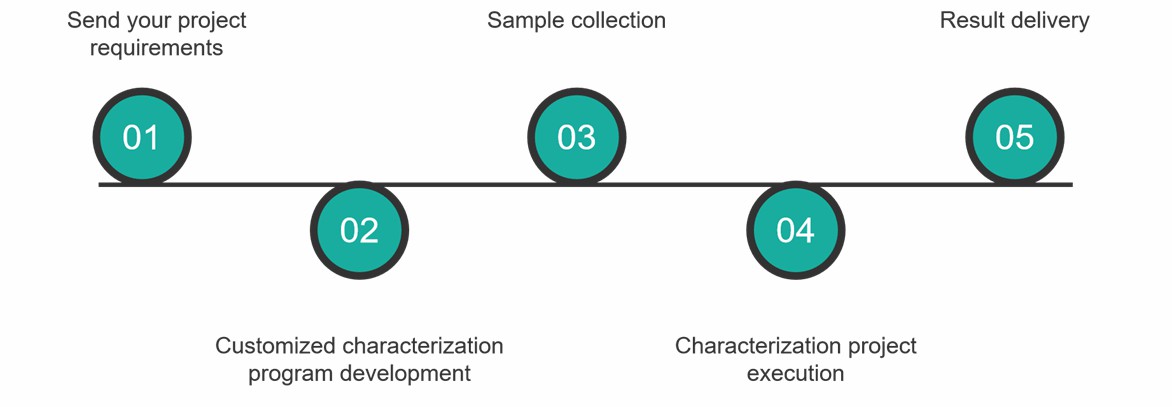
Characterization Service Process
At Creative Proteomics, you can get comprehensive ADCs characterization services by following these simple steps.
Creative Proteomics is your trusted partner in advancing antibody therapeutics. Our expertise, advanced technologies, and customized solutions ensure that you have the critical information you need to optimize the development of your ADCs to improve efficacy, safety, and commercial success. Contact us to learn more about our ADCs analysis and characterization services, and how we can assist you with your drug development demands.


