Vitamins are essential organic compounds that play pivotal roles in sustaining diverse physiological functions within living organisms. These micronutrients are indispensable in minimal amounts, yet wield a profound impact on health and well-being. As our comprehension of the paramount significance of vitamins in preserving optimal health evolves, so does the imperative for precise and exhaustive analysis. At Creative Proteomics, we recognize the critical importance of vitamin detection and extend a comprehensive suite of analysis services to meet this imperative need.
Vitamin Analysis Service at Creative Proteomics
Accurate Identification and Quantification: We identify and quantify a comprehensive range of vitamins present in your samples, ensuring precision and reliability in results.
Plant Vitamin Analysis: Our expertise extends to analyzing vitamins in plants, contributing to agricultural research, dietary planning, and nutritional studies.
Dietary Supplement Analysis: We assess the vitamin content of dietary supplements, ensuring their potency and quality meet regulatory standards.
Vitamin Analysis Techniques
- Triple Quadrupole Mass Spectrometry (e.g., Agilent 6495 Triple Quadrupole LC/MS): This technique offers precise quantification by employing multiple stages of mass analysis, enabling accurate detection of vitamins.
- High-Resolution Mass Spectrometry (e.g., Thermo Scientific Q Exactive HF-X): Our high-resolution mass spectrometers provide exceptional accuracy and resolution, enabling comprehensive detection of a wide range of vitamins.
- Liquid Chromatography (LC) Coupled with Mass Spectrometry (MS): This combination ensures efficient separation and sensitive detection, enhancing the accuracy of vitamin analysis.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Vitamin Analyzed (including but not limited to)
| Vitamin Group |
Vitamins Analyzed |
| Fat-Soluble Vitamins |
A (Retinol), D (Calciferol), E (Tocopherol), K (Phylloquinone) |
| Water-Soluble Vitamins |
B1 (Thiamine), B2 (Riboflavin), B3 (Niacin), B5 (Pantothenic Acid), B6 (Pyridoxine), B7 (Biotin), B9 (Folate), B12 (Cobalamin), C (Ascorbic Acid) |
Applications of Plant Vitamin Analysis
Agricultural Research and Crop Improvement: By analyzing the vitamin content of different plant species, our services support agricultural research aimed at optimizing crop yields, enhancing nutritional value, and developing sustainable farming practices.
Dietary Planning and Nutrition Studies: Our analysis helps nutritionists and researchers assess the vitamin composition of plant-based diets, leading to evidence-based dietary recommendations and improved nutritional guidelines.
Food Product Development: For the food industry, our plant vitamin analysis aids in formulating fortified foods, beverages, and supplements, ensuring accurate vitamin content and meeting regulatory standards.
Pharmaceutical and Herbal Medicine Research: In the pharmaceutical and herbal medicine sectors, our analysis contributes to understanding the vitamin content of medicinal plants, facilitating the development of natural remedies and therapeutic agents.
Nutraceutical and Functional Food Development: Our services support the creation of nutraceuticals and functional foods enriched with specific vitamins, offering consumers enhanced health benefits.
Environmental Studies: Plant vitamin analysis can provide insights into the impact of environmental factors on vitamin content, aiding ecological studies and promoting sustainable environmental practices.
Sample Requirements for Vitamin Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
50 mg - 1 g |
Case 1. Exploring Vitamin D3 Biosynthesis and Response to UV Treatment in Various Plant Species Using LC-APCI-MS/MS
Background:
The study aimed to investigate the presence of vitamin D3 and its precursors in various plant species and assess the impact of UV treatment on their levels. The biosynthesis of vitamin D3 in plants, its potential as a dietary source, and the need for accurate analytical methods were the key motivations for this research
Samples:
A diverse set of plant materials, including Spinacia oleracea L., Solanum glaucophyllum Desf., Solanum lycopersicum L., Sorghum bicolor (L.) Moench, Capsicum annuum L., and Pisum sativum L., were selected for analysis. The study also utilized a reference material, Spinacia oleracea L., as a benchmark.
Methods:
The research employed a comprehensive methodology that involved multiple steps. The following details outline the technical process:
Sample Preparation: The freeze-dried plant material underwent saponification using potassium hydroxide, ethanol, and ascorbic acid to liberate free forms of vitamin D and sterols. An antioxidant (ascorbic acid) and nitrogen flushing were used to prevent oxidation. Silica solid phase extraction was employed to purify the extracts and eliminate interfering substances.
Chromatographic Separation: High-performance liquid chromatography (HPLC) was performed using a Phenomenex Kinetex PFP column with a gradient elution method. The column's selectivity, coupled with selected reaction monitoring (SRM) on a triple quadrupole mass spectrometer, enabled the separation and quantification of analytes.
Mass Spectrometry Analysis: Atmospheric pressure chemical ionization (APCI) was used for mass spectrometry in positive mode. Deuterium-labeled vitamin D3 served as an internal standard for both vitamin D3 and sterols. SRM was employed for quantification, offering improved selectivity and sensitivity. APCI was chosen over ESI due to higher sensitivity for vitamin D and sterols.
Method Validation: The developed method was thoroughly validated for accuracy, linearity, and reproducibility. Blank samples and internal standards were used to assess selectivity and LOD. Standard addition was used to evaluate accuracy, and inter-day reproducibility was determined across different spiking levels.
Results
The study's results indicated varying levels of cholesterol across different plant species, unaffected by UV treatment. Vitamin D3 and its precursor, 7-dehydrocholesterol, were detected in specific plants following UV exposure, suggesting a photolytic reaction akin to animals. However, vitamin D3 synthesis was not uniform among all plant species studied.
In conclusion, the study successfully developed and validated a rapid LC–APCI-MS/MS method for analyzing vitamin D3 and sterols in plant materials. It shed light on the potential biosynthesis of vitamin D3 in plants and its responsiveness to UV treatment, providing a foundation for further research in this area.
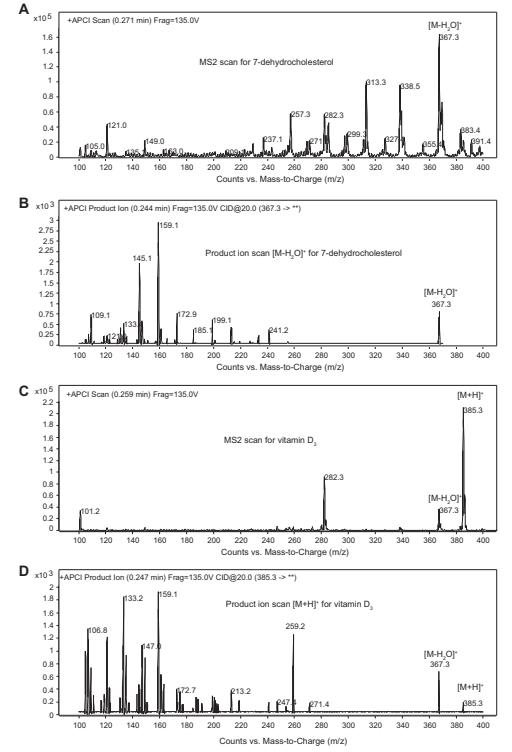 Examples of APCI MS2 scans and product ions scans
Examples of APCI MS2 scans and product ions scans
Reference
- Jäpelt, Rie Bak, et al. "LC–MS/MS with atmospheric pressure chemical ionisation to study the effect of UV treatment on the formation of vitamin D3 and sterols in plants." Food chemistry 129.1 (2011): 217-225.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Examples of APCI MS2 scans and product ions scans
Examples of APCI MS2 scans and product ions scans