Why Analyze Hesperetin?
Hesperetin, a natural flavanone glycoside abundantly found in citrus fruits, has been cited in numerous scientific findings due to its multifaceted health benefits. It possesses powerful antioxidant, anti-inflammatory, anti-carcinogenic, and cardio-protective characteristics, among others. A greater fascination lies in its ability to be a promising therapeutic agent, facilitating the treatment of various conditions such as obesity, diabetes, hypertension, and inflammatory ailments.
With its diverse therapeutic potential, the requirement to analyze hesperetin is explicit. Its accurate analysis reveals integral insights about its structure, behavior, interaction with other biomolecules and the overall mechanism. As a result, these discerning details elucidate how best to utilize this bioactive compound for medicinal, nutritional, and cosmetic applications, and even in the development of novel drugs.
Specific Hesperetin Analysis Offered by Creative Proteomics
Creative Proteomics offers a diverse range of specialized services for hesperetin analysis, tailored to meet the specific needs of clients across various industries. Our comprehensive portfolio includes:
- Quantitative Analysis: Accurate quantification of hesperetin in complex matrices using advanced analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS).
- Qualitative Analysis: Identification and characterization of hesperetin and its derivatives in various samples through comprehensive spectroscopic analysis and chromatographic methods.
- Metabolite Profiling: Investigation of hesperetin metabolism pathways and identification of metabolites using cutting-edge mass spectrometry techniques coupled with data analysis software.
- Bioavailability Studies: Assessment of hesperetin bioavailability in biological samples, including plasma, urine, and tissues, to understand its absorption, distribution, and elimination kinetics.
- Stability Testing: Evaluation of hesperetin stability under different storage conditions, providing critical data for shelf-life determination and formulation optimization.
- Customized Solutions: Tailored analytical approaches and protocols to address specific client requirements, ensuring optimal results and maximum value.
Hesperetin Analysis Techniques
Liquid Chromatography-Mass Spectrometry (LC-MS): High-resolution LC-MS systems, such as the Thermo Scientific™ Q Exactive™ series, enable sensitive and accurate analysis of hesperetin and its metabolites in complex biological matrices.
Gas Chromatography-Mass Spectrometry (GC-MS): Advanced GC-MS instruments, including the Agilent 7890B GC system coupled with the Agilent 5977A Mass Selective Detector, facilitate the analysis of volatile compounds and derivatized hesperetin derivatives with exceptional sensitivity and specificity.
High-Performance Liquid Chromatography (HPLC): Cutting-edge HPLC systems equipped with UV-Vis and fluorescence detectors, such as the Agilent 1260 Infinity II series, enable precise quantification and separation of hesperetin from interfering compounds in complex samples.
Nuclear Magnetic Resonance (NMR) Spectroscopy: State-of-the-art NMR spectrometers, such as the Bruker AVANCE NEO series, provide valuable structural information for elucidating the chemical properties and molecular interactions of hesperetin and its derivatives.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Hesperetin Assay
| Sample Type |
Recommended Sample Volume |
| Pure Hesperetin |
1-5 mg |
| Hesperetin Standard |
1-10 mg |
| Citrus Extract |
100-500 mg |
| Serum/Plasma |
100-500 μL |
| Urine |
0.5-2 mL |
| Tissue Homogenate |
10-50 mg |
| Dietary Supplement |
100-500 mg |
| Food Product |
5-10 g |
Deliverables of Hesperetin Analysis
At Creative Proteomics, we ensure our clients receive exhaustive and accurate results. With our hesperetin analysis services, we provide:
- A full report of our comprehensive experimental process
- Detailed analysis results including chromatograms, spectrometry, and identification data
- Expert interpretation and suggestions for future research
Applications of Hesperetin Analysis
Pharmaceutical Development: Hesperetin analysis guides drug discovery and development efforts, optimizing formulations for various medical conditions such as cancer, cardiovascular diseases, and inflammation.
Nutritional Science: Analysis contributes to understanding hesperetin's bioavailability, metabolism, and physiological effects, shedding light on its role in human nutrition and health.
Biomedical Investigations: Hesperetin analysis facilitates biomedical research focused on elucidating its mechanisms of action and therapeutic potential, particularly in areas such as neuroprotection, antimicrobial activity, and bone health.
Cosmeceuticals and Personal Care Products: Researchers confirm hesperetin presence and efficacy in skincare formulations, supporting claims related to skin protection, anti-aging, and complexion enhancement.
Anti-inflammatory Agents: Analysis aids in the development of anti-inflammatory agents for conditions such as arthritis, asthma, and inflammatory bowel disease.
Cardioprotective Agents: Hesperetin analysis contributes to formulating cardiovascular supplements and medications aimed at reducing the risk of heart disease and stroke.
Neuroprotective Compounds: Researchers investigate hesperetin's neuroprotective properties and potential applications in neurodegenerative diseases like Alzheimer's and Parkinson's disease.
Antimicrobial Agents: Analysis supports the development of antimicrobial agents for applications in food preservation, agricultural products, and industrial processes.
Anticancer Therapies: Researchers explore novel anticancer therapies leveraging hesperetin's mechanisms of action and synergistic effects with conventional chemotherapy agents.
Pharmacokinetics and Drug Delivery: Hesperetin analysis aids in studying its absorption, distribution, metabolism, and excretion properties, informing optimal drug delivery strategies.
Oxidative Stress and Antioxidant Activity: Analysis contributes to research on oxidative stress-related diseases and antioxidant interventions, evaluating hesperetin's ability to scavenge free radicals and protect against cellular damage.
Inflammation and Immune Modulation: Researchers investigate hesperetin's anti-inflammatory and immunomodulatory effects, exploring its potential therapeutic applications in immune-related disorders and inflammatory diseases.
Metabolic Syndrome and Obesity: Analysis supports research on hesperetin's effects on lipid metabolism, insulin sensitivity, and glucose homeostasis, offering insights into its potential as an adjunct therapy for managing metabolic syndrome and obesity-associated complications.
Cancer Biology and Chemoprevention: Hesperetin analysis aids in understanding its mechanisms of action against cancer initiation, progression, and metastasis, paving the way for novel chemopreventive and anticancer therapies.
Case. Development and Validation of a LC-MS/MS Method for Quantification of Hesperetin and Its Enantiomers in Human Plasma and Urine
Background
Hesperidin, a flavonoid found abundantly in citrus fruits, holds promise for various health benefits, including potential effects on osteoporosis and vascular protection. Its bioactivity is influenced by its enantiomers, necessitating sensitive analytical methods for quantification. Existing methods for hesperetin quantification lacked the precision required for plasma analysis, prompting the need for a more sensitive approach.
Sample
Human plasma and urine samples were collected from volunteers who consumed a dose of purified hesperetin-7-O-glucoside. Plasma samples were collected at various time points up to 24 hours post-consumption, while urine samples were collected over a 24-hour period.
Technical Method
Sample Preparation:
- Flavanone-free plasma and urine samples were spiked with hesperetin and internal standard (−)-homoeriodictyol.
- Samples were mixed with 10% acetic acid solution and incubated with β-glucuronidase/sulfatase overnight at 37°C.
- Hydrolyzed samples were diluted with 0.1% formic acid solution and loaded onto solid-phase extraction cartridges (Waters Oasis® MAX 96-Well Plate) preconditioned with methanol and water.
Chromatographic Separation:
- Hesperetin was analyzed using reversed-phase ultra-performance liquid chromatography (UPLC) with an Acquity UPLC HSS T3 column.
- Hesperetin enantiomers were separated using a DAICEL Chiralpak® IA column.
- Solvent systems composed of water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B) were used.
- Gradient elution conditions were optimized for each column to achieve efficient separation.
Mass Spectrometry Detection:
- A Waters Acquity UPLC system coupled with a Quattro micro API mass spectrometer detector equipped with an electron spray ionization source was utilized.
- The mass spectrometer was operated in negative ion mode.
- Molecular transitions m/z 301.0 to m/z 163.8 and to m/z 150.8 were monitored for quantification of hesperetin and internal standard, respectively.
Data Processing:
- MassLynx software was used for data acquisition and processing.
- Peak areas of hesperetin and internal standard were measured for quantification.
Validation:
- The method was validated for linearity, precision, accuracy, and sensitivity.
- Calibration curves were constructed using weighted linear regression models.
- The limit of quantification (LoQ) was determined based on FDA guidelines.
- Trueness was evaluated by recovery studies at different concentrations.
- Precision was assessed by calculating repeatability (r) and intermediate reproducibility (iR) values.
Results
The method was validated for linearity, precision, accuracy, and sensitivity. Linearity was assessed by analyzing different concentrations of racemic hesperetin in plasma and urine. The limit of quantification (LoQ) was determined based on FDA guidelines, and the method exhibited high trueness and precision. The validated method was successfully applied to quantify hesperetin and its enantiomers in human plasma and urine samples from volunteers after hesperetin-7-O-glucoside consumption.
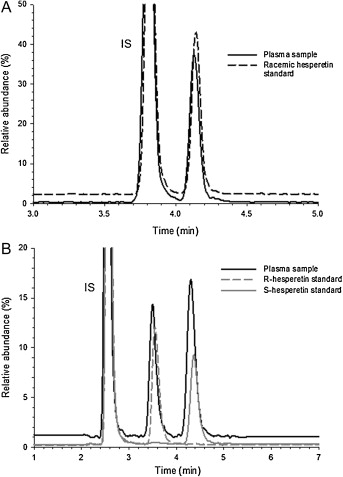 UPLC–MS/MS chromatograms (MRM experiment) of racemic hesperetin and (−)-homoeriodictyol (IS) (A) separated on a UPLC HSS T3 column and of purified hesperetin R- and S-enantiomers and IS (B) separated on a DAICEL Chiralpak® IA-3 column.
UPLC–MS/MS chromatograms (MRM experiment) of racemic hesperetin and (−)-homoeriodictyol (IS) (A) separated on a UPLC HSS T3 column and of purified hesperetin R- and S-enantiomers and IS (B) separated on a DAICEL Chiralpak® IA-3 column.
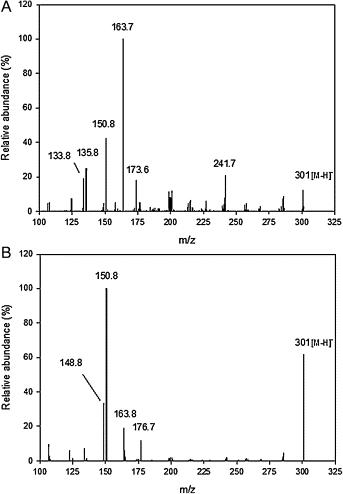 MS/MS spectra of racemic hesperetin standard solution (A) and (−)-homoeriodictyol (IS) solution (B) obtained in negative ion mode.
MS/MS spectra of racemic hesperetin standard solution (A) and (−)-homoeriodictyol (IS) solution (B) obtained in negative ion mode.
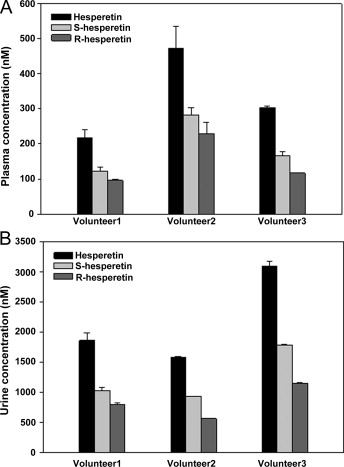 Concentrations of racemic hesperetin and hesperetin enantiomers in human plasma at Cmax (A) and urine (B) for three volunteers fed with 19 mg of hesperetin-7-glucoside.
Concentrations of racemic hesperetin and hesperetin enantiomers in human plasma at Cmax (A) and urine (B) for three volunteers fed with 19 mg of hesperetin-7-glucoside.
Reference
- Lévèques, Antoine, et al. "UPLC–MS/MS quantification of total hesperetin and hesperetin enantiomers in biological matrices." Journal of pharmaceutical and biomedical analysis 57 (2012): 1-6.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service UPLC–MS/MS chromatograms (MRM experiment) of racemic hesperetin and (−)-homoeriodictyol (IS) (A) separated on a UPLC HSS T3 column and of purified hesperetin R- and S-enantiomers and IS (B) separated on a DAICEL Chiralpak® IA-3 column.
UPLC–MS/MS chromatograms (MRM experiment) of racemic hesperetin and (−)-homoeriodictyol (IS) (A) separated on a UPLC HSS T3 column and of purified hesperetin R- and S-enantiomers and IS (B) separated on a DAICEL Chiralpak® IA-3 column. MS/MS spectra of racemic hesperetin standard solution (A) and (−)-homoeriodictyol (IS) solution (B) obtained in negative ion mode.
MS/MS spectra of racemic hesperetin standard solution (A) and (−)-homoeriodictyol (IS) solution (B) obtained in negative ion mode. Concentrations of racemic hesperetin and hesperetin enantiomers in human plasma at Cmax (A) and urine (B) for three volunteers fed with 19 mg of hesperetin-7-glucoside.
Concentrations of racemic hesperetin and hesperetin enantiomers in human plasma at Cmax (A) and urine (B) for three volunteers fed with 19 mg of hesperetin-7-glucoside.