Plant lipidomics entails the comprehensive analysis of lipid species within plant organisms, encompassing a wide array of compounds such as glycerolipids, phospholipids, sterols, and sphingolipids. Lipids, being essential components of cellular membranes, serve as reservoirs of energy and contribute to cell signaling processes. The significance of plant lipidomics lies in its ability to unravel the intricate web of lipid metabolism and its impact on plant growth, development, and stress responses.
Plant lipidomics provides valuable insights into:
Biosynthetic Pathways: By identifying and quantifying lipid species, researchers can decipher the various biosynthetic pathways involved in lipid metabolism, shedding light on the enzymes and intermediates participating in lipid synthesis.
Stress Responses: Changes in lipid composition can serve as indicators of plant responses to environmental stresses. Lipidomics aids in understanding how plants adapt to challenges such as drought, salinity, and temperature fluctuations.
Nutrient Allocation: Lipidomics helps elucidate how lipids are distributed among different plant tissues and organs, offering clues about nutrient allocation and storage strategies.
Secondary Metabolism: Lipids are precursors to many secondary metabolites with roles in defense against herbivores, pathogens, and abiotic stresses. Plant lipidomics contributes to the study of these defense mechanisms.
Plant Lipidomics Service at Creative Proteomics
At Creative Proteomics, we understand the diverse research needs within the realm of plant lipidomics. As such, we offer a range of specialized projects that cater to various aspects of lipid analysis, addressing the complexities of lipid metabolism in plants.
Lipid Profiling and Quantitative Analysis
Our lipid profiling projects involve the comprehensive analysis of lipid species present in different plant tissues and organs. We employ high-resolution mass spectrometry techniques, such as liquid chromatography-mass spectrometry (LC-MS), to precisely quantify lipid species and analyze their relative abundance. This approach provides invaluable insights into the dynamic changes of lipid composition under various physiological and environmental conditions.
Lipid Identification and Structural Characterization
Identifying and characterizing lipid species is paramount to understanding their functional roles in plant biology. Creative Proteomics employs advanced mass spectrometry, including matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) and gas chromatography-mass spectrometry (GC-MS), to accurately identify lipid molecules and elucidate their structural characteristics. This detailed analysis contributes to deciphering the intricate pathways of lipid metabolism.
Temporal and Spatial Dynamics of Lipidomics
The temporal and spatial dynamics of lipid metabolism play a pivotal role in plant growth, development, and responses to stress. Creative Proteomics offers dynamic lipidomics projects that investigate how lipid composition changes over time or in specific plant tissues. This approach provides a comprehensive view of lipid metabolism and its regulatory mechanisms.
Plant Lipidomics Analytical Techniques
Creative Proteomics is equipped with state-of-the-art instrumentation and technology that underpin our plant lipidomics service. Our analytical techniques are designed to deliver accurate, high-resolution results that contribute to advancing your research endeavors.
- High-Resolution Mass Spectrometry
Our lipidomics analysis relies on high-resolution mass spectrometry platforms, including the esteemed Thermo Scientific Q Exactive series. The Q Exactive HF-X mass spectrometer, with its exceptional resolution and sensitivity, allows us to precisely quantify and identify lipid species, even in complex plant matrices.
- Gas Chromatography-Mass Spectrometry (GC-MS)
For volatile lipid analysis, such as fatty acids, we employ the Agilent 7890B gas chromatography system coupled with a mass spectrometer (e.g., Agilent 5977A). This powerful combination enables the separation and identification of a wide range of lipid species, contributing to a comprehensive understanding of lipid composition.
- Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS)
Creative Proteomics harnesses the capabilities of MALDI-MS to directly analyze intact lipids from plant samples. This technique offers rapid and sensitive profiling of lipid species, enhancing our ability to capture lipidomic diversity.
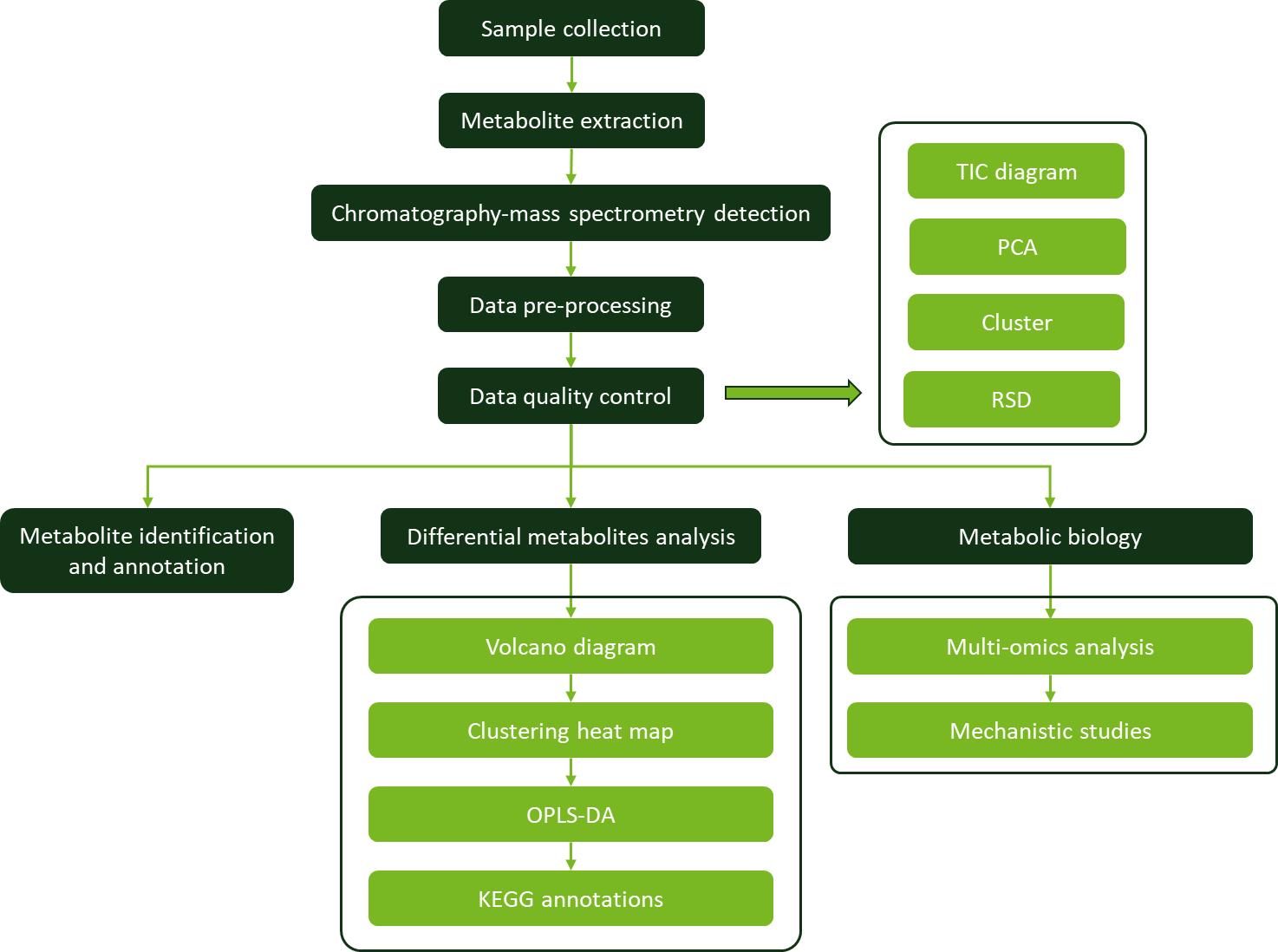 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Plant Lipidomics Compounds Analyzed (including but not limited to)
| Types |
Compounds |
| Glycerolipids |
Monogalactosyldiacylglycerol (MGDG), Digalactosyldiacylglycerol (DGDG), Triacylglycerol (TAG), Phosphatidic Acid (PA), Diacylglycerol (DAG), Sulfoquinovosyldiacylglycerol (SQDG), Phosphatidylglycerol (PG), Phosphatidylinositol (PI), Phosphatidylserine (PS), Phosphatidylethanolamine (PE) |
| Phospholipids |
Phosphatidylcholine (PC), Phosphatidylinositol Phosphate (PIP), Phosphatidylglycerophosphate (PGroP), Phosphatidylglyceroside (PGroS), Phosphatidylmethylethanolamine (PME), Phosphatidylmethanol (PMT) |
| Sterols |
β-Sitosterol, Campesterol, Stigmasterol, Ergosterol, Brassicasterol, Delta-5-Avenasterol, Stigmastanol, Campestanol |
| Sphingolipids |
Glucosylceramide, Ceramide, Sphingomyelin, Phytosphingosine, Ceramide-1-phosphate (C1P), Sphingosine-1-phosphate (S1P), Inositolphosphorylceramide (IPC) |
| Fatty Acids |
Palmitic Acid (C16:0), Oleic Acid (C18:1), Linoleic Acid (C18:2), α-Linolenic Acid (C18:3), Stearic Acid (C18:0), Arachidonic Acid (C20:4), Behenic Acid (C22:0) |
| Isoprenoids |
Geranylgeranyl Pyrophosphate (GGPP), Farnesyl Pyrophosphate (FPP), Geranylgeraniol, Squalene, Phytyl Diphosphate, Carotenoids (e.g., β-Carotene, Lutein, Zeaxanthin) |
| Other Lipid Classes |
Betaine Lipids, Sulfolipids, Lysolipids, Oxylipins, Lipoxygenase-Derived Lipids, Eicosanoids (e.g., Prostaglandins, Leukotrienes) |
Sample Requirements for Plant Lipidomics Compounds Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
50 mg - 1 g |
| Cell Samples |
Cells and Culture |
106 - 108 cells |
Case 1. Exploring Marine Macrophyte Lipidomics: Innovative Mass Spectrometry Analysis and Bioactive Discoveries
Background:
The marine environment boasts diverse habitats, nurturing a wealth of life forms. Within this context, marine macrophytes, spanning from microalgae to macroalgae and seagrasses, hold untapped potential for bioactive compounds. These organisms, including glycolipids, phospholipids, and betaine lipids, present promising antioxidant, anti-inflammatory, and antimicrobial properties. Advanced mass spectrometry techniques enable the comprehensive exploration of their lipid composition. This study examines the lipid profiles of marine macrophytes, novel analytical strategies, and the potential health benefits of these compounds, showcasing their role in medicine, nutraceuticals, and the food industry.
Samples:
The study focuses on a diverse range of marine macrophytes, including both macroalgae and halophytes. These organisms are sourced from various marine habitats, such as tidal saltmarshes, coastal lagoons, and mangroves. The selected macrophytes represent a cross-section of species known for their bioactive potential and are carefully collected and processed for subsequent lipidomic analysis.
Methods:
The analysis of the lipidomic composition of marine macrophytes entails a comprehensive methodology centered around advanced mass spectrometry (MS) techniques. To capture the diverse lipid classes present within these organisms, a multi-step process is employed, involving lipid extraction, chromatographic separation, and mass spectrometric detection.
Lipid Extraction: The lipid extraction process involves carefully harvesting the marine macrophytes and subjecting them to lipid extraction procedures. These procedures are designed to isolate the lipid components from the bulk biomass, creating a lipid extract that encompasses a wide array of lipid classes, including glycolipids, phospholipids, and betaine lipids.
Chromatographic Separation: The extracted lipids are subsequently subjected to chromatographic separation, enabling the isolation of distinct lipid classes for further analysis. Different chromatographic techniques, such as thin-layer chromatography (TLC), gas chromatography (GC), and liquid chromatography (LC), are utilized based on the specific lipid classes under investigation. GC and LC are often coupled with mass spectrometry (GC-MS and LC-MS, respectively) to enhance the separation and detection of lipid species.
Mass Spectrometric Analysis: The heart of the methodology lies in the mass spectrometric analysis, which involves the ionization and fragmentation of lipid species to generate mass spectra. This process provides detailed information about the molecular composition and structure of the identified lipids. MS techniques, such as electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), are employed to ionize the lipid molecules. Tandem mass spectrometry (MS/MS) is often utilized to further elucidate the structural details of the identified lipids by subjecting them to controlled fragmentation.
Results
The study reveals intricate lipid profiles of marine macrophytes, highlighting the presence of various lipid classes such as glycerolipids (MGDG, DGDG), sulfoquinovosyl diglycerides (SQDG), betaine lipids, phospholipids (PC, PE, PI, PS), and others. MS/MS fragmentation patterns provide insights into lipid structural details, confirming the identification of lipid classes and their constituent molecules. Shotgun lipidomics and LC-MS approaches unveil over 200 lipid species from 12 lipid classes in certain macrophyte samples. Statistical analyses contribute to understanding lipidomic alterations under different conditions, offering potential insights into the bioactivity of specific lipid species.
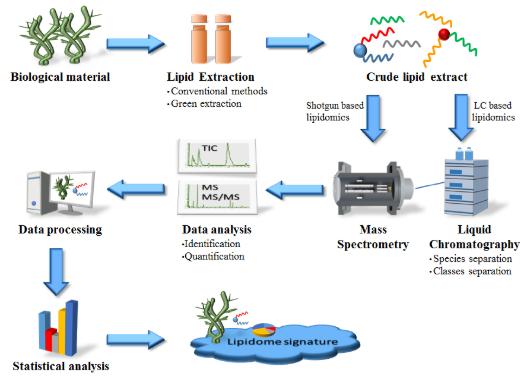 MS-based lipidomics to screen bioactive lipids from marine macrophytes (Maciel et al., 2016).
MS-based lipidomics to screen bioactive lipids from marine macrophytes (Maciel et al., 2016).
Reference
- Maciel, Elisabete, et al. "Bioprospecting of marine macrophytes using MS-based lipidomics as a new approach." Marine drugs 14.3 (2016): 49.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service MS-based lipidomics to screen bioactive lipids from marine macrophytes (Maciel et al., 2016).
MS-based lipidomics to screen bioactive lipids from marine macrophytes (Maciel et al., 2016).