What are Carotenoids?
Carotenoids are a class of natural pigments synthesized by plants, algae, and some bacteria. These compounds are responsible for the vibrant red, orange, and yellow hues observed in various fruits, vegetables, and flowers. Structurally, carotenoids consist of a polyene chain with alternating single and double bonds, conjugated with end groups such as cyclic rings or ketones. This unique structure allows carotenoids to absorb light in the visible spectrum, contributing to their vivid colors.
There are over 700 known carotenoids, classified into two major groups: carotenes and xanthophylls. Carotenes are hydrocarbons and include well-known compounds such as β-carotene, α-carotene, and lycopene. Xanthophylls, on the other hand, contain oxygen atoms and include compounds like lutein, zeaxanthin, and β-cryptoxanthin. Each carotenoid has a distinct color and plays specific biological roles in different organisms.
Analyzing carotenoids is crucial for understanding their functions, biosynthesis pathways, and potential applications in various industries. Carotenoids serve as antioxidants, protecting organisms from oxidative damage caused by reactive oxygen species. Additionally, these pigments play a critical role in photosynthesis, capturing light energy and transferring it to chlorophyll molecules.
Carotenoids also have significant nutritional value. In humans, certain carotenoids are converted into vitamin A, an essential nutrient for vision, growth, and immune function. Moreover, carotenoids have been associated with a reduced risk of chronic diseases, including certain types of cancer, cardiovascular diseases, and age-related macular degeneration. Understanding the composition and levels of carotenoids in different foods and supplements is crucial for developing strategies to enhance their health benefits.
Creative Proteomics: Carotenoid Metabolism Analysis Projects
Carotenoid Profiling: Our comprehensive carotenoid profiling service enables the identification and quantification of carotenoids in various samples, including plants, algae, and microorganisms. By employing high-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS), we can accurately analyze and characterize a wide range of carotenoids, providing detailed information about their composition and abundance.
Metabolic Pathway Analysis: Creative Proteomics specializes in elucidating carotenoid biosynthesis pathways in different organisms. Through a combination of targeted and untargeted metabolomics approaches, we can identify key intermediates, enzymes, and regulatory factors involved in carotenoid metabolism. This knowledge is invaluable for understanding the regulation of carotenoid production and potentially engineering pathways to enhance carotenoid content in crops.
Stability and Degradation Studies: Carotenoids are susceptible to degradation due to various environmental factors, such as light, heat, and oxygen. Creative Proteomics offers stability and degradation studies to assess the effects of these factors on carotenoid stability and identify degradation products. This information can aid in the development of proper storage and processing techniques to preserve carotenoid content in food and cosmetic products.
Carotenoid Analysis Techniques
- High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS): This technique combines the separation power of HPLC with the detection and identification capabilities of MS. It allows for the accurate separation and quantification of carotenoids in complex samples, providing high-resolution data on their molecular structures and concentrations. Instruments such as Agilent 1290 Infinity II LC and Thermo Scientific Q Exactive series MS are commonly utilized for carotenoid analysis.
- Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is another powerful technique employed in carotenoid analysis. It involves the separation of volatile carotenoid compounds by gas chromatography, followed by their detection and characterization using mass spectrometry. Instruments such as Agilent 7890B GC and Waters Xevo TQD MS are commonly used in this context.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Carotenoids Analyzed (including but not limited to)
Carotenes
| β-Carotene |
α-Carotene |
Lycopene |
γ-Carotene |
ζ-Carotene |
| Neurosporene |
Phytoene |
Phytofluene |
Prolycopene |
β-Carotene-5,6-epoxide |
| β-Carotene-9',10'-epoxide |
δ-Carotene |
|
|
|
Xanthophylls
| Lutein |
Zeaxanthin |
β-Cryptoxanthin |
Astaxanthin |
Canthaxanthin |
| Violaxanthin |
Neoxanthin |
Antheraxanthin |
Capsanthin |
Capsorubin |
| Zeinoxanthin |
Rubixanthin |
Cryptoflavin |
Adonixanthin |
Prasinoxanthin |
| Oscillaxanthin |
Echinenone |
Loliolide |
Norbixin |
Auroxanthin |
| Sarcinaxanthin |
Myxoxanthophyll |
Diadinoxanthin |
Diatoxanthin |
Prasinoxanthin |
| Fucoxanthin |
Peridinin |
Dinoxanthin |
Diadinoxanthin |
|
Norisoprenoids
Why Choose Us?
- High-throughput, multiple types of carotenoid markers available
- High sensitivity, detection accuracy down to the ng level
- Absolute quantification of standard samples, providing standard curves for each compound
Sample Requirements for Carotenoids Assay
| Sample Types |
Minimum Sample Size |
Biological Repeat |
| Plant Samples |
Stem, bud, node, leaf, flower, fruit, root, healing tissue |
600mg |
3-6 |
Case 1. Development of Analytical Method for Fat-Soluble Vitamins and Carotenoids in Avocado Fruit Using LC/MS
Background:
The background of this study is the development of an analytical method for the analysis of fat-soluble vitamins and carotenoids in avocado fruit.
Sample:
Three different varieties of avocados were collected from Costa Rica farms: Simmonds, Guatemala, and Hass. Each variety was analyzed in three batches, with different maturation periods.
Technical Platform and Procedure:
Reagents: The reagents used in the study were tert-Butyl methyl ether, methanol, potassium hydroxide, and various standards of fat-soluble vitamins and carotenoids.
Sample Treatment and Preparation: Freeze-dried avocado samples were homogenized, and chloroform was added for extraction. The extraction was repeated twice, and the lipid fraction was obtained through evaporation. The lipid fraction was then subjected to saponification.
Optimization of Saponification Conditions: The conditions for the saponification reaction were optimized by varying the base concentration and temperature. The conditions that provided the highest recovery of analytes were selected for further analysis.
Sample Saponification: A portion of the lipid fraction was mixed with KOH in ethanol and pyrogallol and saponified at 80 °C. The resulting mixture was cooled, and a liquid-liquid extraction using hexane was performed.
Stationary Phase and Selection of Chromatographic Conditions: Different chromatographic columns and mobile phases were tested to achieve optimal separation of the analytes. A C30 column with a mobile phase of methanol and MTBE was selected.
Chromatographic Conditions: The analysis was performed using an LC/MS system with gradient elution and a constant flow rate. The column temperature was maintained at 10.0 ± 0.8 °C.
MS Detection System Conditions: The mass spectrometer was used for fragmentation and detection of the analytes. Different ionization modes and monitoring techniques were employed to enhance sensitivity and specificity.
Results
The optimized method allowed for the simultaneous analysis of fat-soluble vitamins and carotenoids in avocado fruit. The method demonstrated accurate, sensitive, robust, and specific detection of the target compounds. The use of organic solvents and the C30 column provided a versatile approach for analyzing other pigments present in avocados. Saponification was found to be crucial for the recovery of fat-soluble compounds. The method can be extended to analyze fat-soluble vitamins and carotenoids in other matrices. Mass spectrometry played a vital role in the differentiation of structurally similar compounds, such as tocopherol isomers, in avocados.
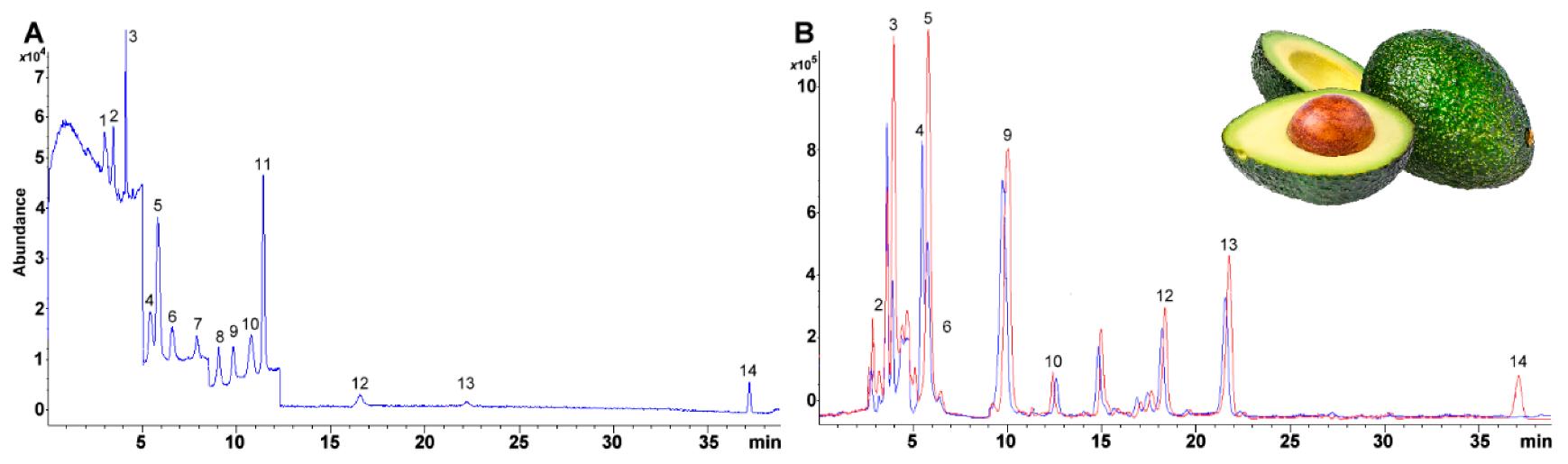 Selected ion monitoring (SIM) chromatogram
Selected ion monitoring (SIM) chromatogram
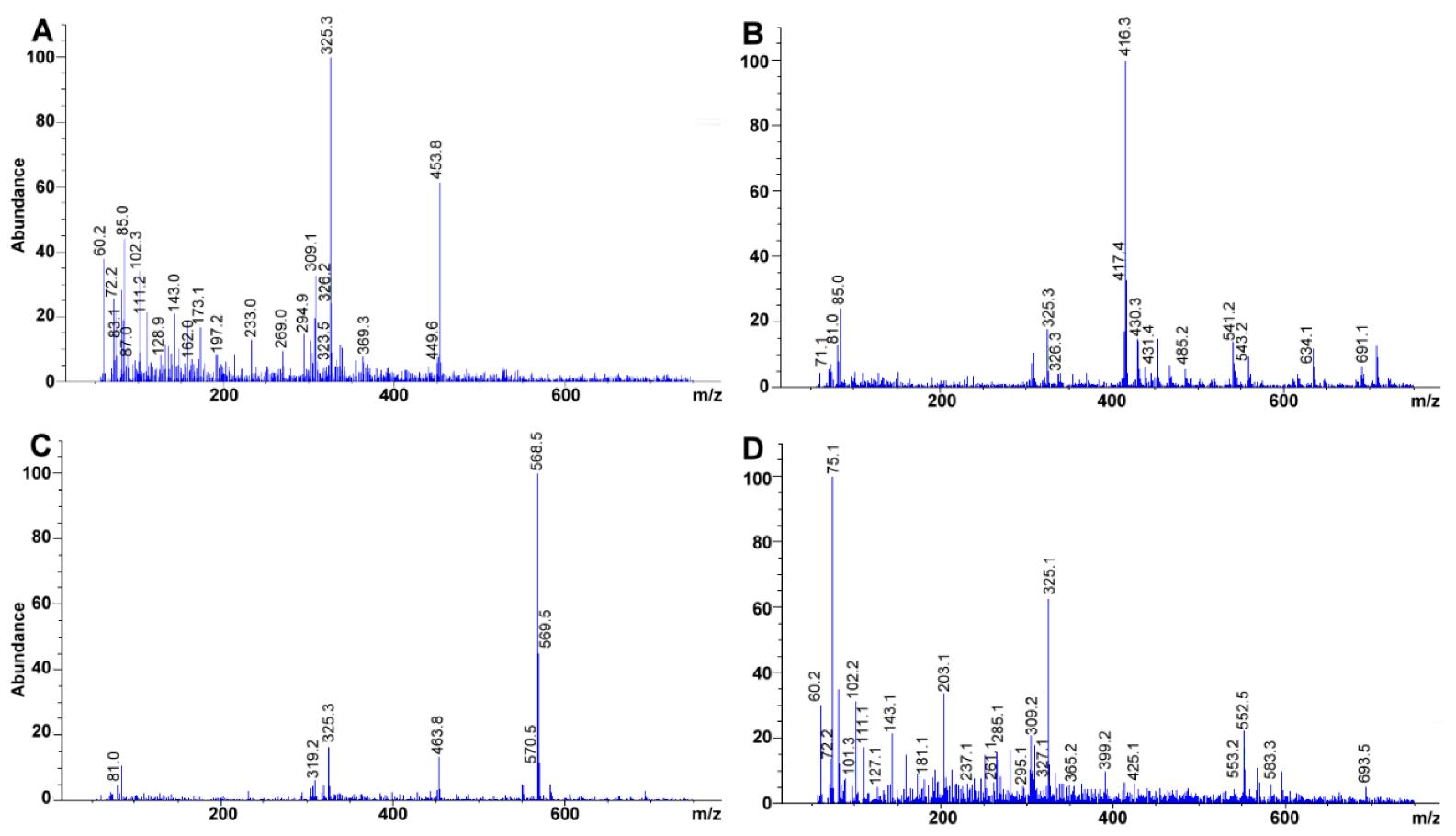 Experimental mass spectra obtained for (A) retinyl acetate, (B) γ-tocopherol, (C) Lutein, (D) β-cryptoxanthin. Total ion chromatograms at 100 mg L−1 each.
Experimental mass spectra obtained for (A) retinyl acetate, (B) γ-tocopherol, (C) Lutein, (D) β-cryptoxanthin. Total ion chromatograms at 100 mg L−1 each.
Reference
- Cortés-Herrera, Carolina, et al. "Simultaneous LC/MS analysis of carotenoids and fat-soluble vitamins in Costa Rican avocados (Persea americana Mill.)." Molecules 24.24 (2019): 4517.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Selected ion monitoring (SIM) chromatogram
Selected ion monitoring (SIM) chromatogram Experimental mass spectra obtained for (A) retinyl acetate, (B) γ-tocopherol, (C) Lutein, (D) β-cryptoxanthin. Total ion chromatograms at 100 mg L−1 each.
Experimental mass spectra obtained for (A) retinyl acetate, (B) γ-tocopherol, (C) Lutein, (D) β-cryptoxanthin. Total ion chromatograms at 100 mg L−1 each.