What are Carbohydrates and Carbohydrate Metabolism?
Carbohydrates, also known as saccharides, are essential organic compounds composed of carbon, hydrogen, and oxygen atoms. They play a crucial role as the primary source of energy and participate in various cellular processes, including cell signaling, structural support, and modulation of the immune response. The chemical structure of carbohydrates categorizes them into monosaccharides, disaccharides, oligosaccharides, and polysaccharides, each with distinct characteristics and biological functions.
Carbohydrate metabolism involves a series of biochemical processes responsible for synthesizing, breaking down, and converting carbohydrates into usable forms of energy. These metabolic pathways occur within cells and rely on specific enzymatic reactions. Understanding carbohydrate metabolism is vital for gaining insights into the mechanisms underlying energy production, glycosylation, and the regulation of various cellular processes.
Carbohydrates Metabolomics Service Provided by Creative Proteomics
1. Quantitative Analysis of Carbohydrate Metabolites:
Creative Proteomics employs advanced mass spectrometry-based techniques to quantitatively analyze a wide range of carbohydrate metabolites. This project utilizes high-resolution mass spectrometry systems such as the Thermo Scientific Orbitrap Fusion Lumos Tribrid Mass Spectrometer and the Waters SYNAPT XS QTOF Mass Spectrometer. These instruments offer exceptional sensitivity, resolution, and mass accuracy, enabling precise quantification of carbohydrate metabolites.
2. Stable Isotope Labeling Analysis:
Creative Proteomics utilizes stable isotope labeling analysis to study the metabolic fluxes within carbohydrate metabolism pathways. This project involves the use of stable isotope-labeled substrates and advanced mass spectrometry systems such as the Agilent 6550 iFunnel Q-TOF LC/MS and the Bruker maXis II Q-TOF Mass Spectrometer. These instruments provide high-resolution and accurate mass measurements, facilitating the tracking of stable isotope-labeled metabolites and metabolic dynamics.
3. Targeted Carbohydrate Metabolite Profiling:
Creative Proteomics offers targeted analysis of specific carbohydrate metabolites of interest. This project utilizes highly selective techniques such as multiple reaction monitoring (MRM) on triple quadrupole mass spectrometers. For example, Creative Proteomics employs the SCIEX Triple Quad™ 6500+ LC-MS/MS System for sensitive and specific quantification of target carbohydrate metabolites. This targeted approach enables researchers to focus on specific metabolic pathways or biomarkers, providing valuable insights into disease mechanisms and therapeutic targets.
4. Metabolic Pathway Mapping:
Creative Proteomics integrates metabolomics data with metabolic pathway databases to provide comprehensive metabolic pathway mapping for carbohydrate metabolism. This project utilizes data analysis software such as MetaboAnalyst to visualize and understand the interconnectedness of metabolic pathways. This analysis assists researchers in identifying key regulatory nodes and understanding the metabolic network of carbohydrate metabolism.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Carbohydrates and Carbohydrate Metabolites Analyzed (including but not limited to)
Monosaccharides Analysis
| Glucose |
Fructose |
Galactose |
Mannose |
Ribose |
| Xylose |
Arabinose |
Fucose |
Rhamnose |
|
Disaccharides Analysis
| Sucrose |
Lactose |
Maltose |
Trehalose |
Cellobiose |
| Isomaltose |
Melezitose |
Sophorose |
Melibiose |
Gentiose |
| Rutinose |
|
|
|
|
Polysaccharides Analysis
| Starch |
Glycogen |
Cellulose |
Chitin |
Agarose |
| Xylan |
Pectin |
Inulin |
Chondroitin sulfate |
|
Other Carbohydrate Metabolites Analysis
| Glucosamine |
N-Acetylglucosamine |
Sorbitol |
Mannitol |
Mannooligosaccharides (MOS) |
| Glucosamine-6-phosphate (GlcN-6P) |
Glucuronic acid |
Galacturonic acid |
Gluconic acid |
Maltotriose |
| Trehalulose |
Glycerol |
Ethanol |
Xylitol |
Raffinose |
| Galactosamine |
Glycogenin |
Alginate |
Chondroitin |
Heparin |
| Heparan sulfate |
Hyaluronic acid |
|
|
|
Why Choose Us?
- Precise quantification: Absolute quantification of internal standard, linear relationship of the standard curve reaches more than 0.99.
- High sensitivity: Derivatization treatment, excluding impurity interference, GC-MS/MS, sensitivity up to ng/mL level.
Why need Carbohydrates Analysis?
Crop Improvement: By analyzing carbohydrate metabolites and metabolic pathways, researchers can identify key regulatory steps and manipulate them to enhance crop yield, quality, and stress tolerance.
Photosynthesis and Carbon Fixation: Carbohydrate metabolism is closely linked to photosynthesis and carbon fixation in plants. Analyzing carbohydrate metabolites allows researchers to study the efficiency of these processes and gain insights into factors that influence carbon assimilation, allocation, and utilization.
Storage Organs and Sink-Source Relationships: Carbohydrate metabolism analysis helps unravel the dynamics of carbohydrate allocation between storage organs (e.g., tubers, fruits, and seeds) and actively growing tissues (source organs) in plants. Investigating these sink-source relationships is crucial for optimizing crop productivity, as it impacts the accumulation of storage reserves and the distribution of energy and nutrients throughout the plant.
Understanding Cellular Energy Production: Analyzing the intermediates and end products of carbohydrate metabolism provides valuable information about energy production pathways, such as glycolysis, gluconeogenesis, and the citric acid cycle.
Biomarker Discovery: Changes in carbohydrate metabolism are often associated with various diseases, including diabetes, cancer, and metabolic disorders. By analyzing carbohydrate metabolites, researchers can identify potential biomarkers.
Sample Requirements for Carbohydrates Metabolites Assay
| Sample Types |
Minimum Sample Size |
Biological Repeat |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
600mg |
3-6 |
| Animal Samples |
Blood/plasma, urine, tissues (liver, muscle, adipose, etc.), brain tissue, feces, saliva, tears, salivary gland secretions, amniotic fluid, milk, ovarian/testicular tissues, placental tissue, scales/squamous cells, bone marrow. |
500mg/100 μL |
Humans >30/group
Animals 8-10/group |
| Cell Samples |
Adherent cells, cell cultures |
1×107 |
Humans >30/group
Animals 8-10/group |
Case 1. Dynamic Carbohydrate Metabolism during Bulblet Formation and Development in Lilies (Lilium spp.)
Background:
In this study, the focus was on carbohydrate metabolism during the formation and development of bulblets in lilies (Lilium spp.). Lilies are one of the major bulbous flowers in the floriculture industry, and their bulbs play a crucial role in nutrient storage and energy supply for plant growth and reproduction.
Sample:
The study utilized fresh bulbs of L. davidii var. unicolor, a specific variety known for its large size, white and thick flesh, sweet taste, and ornamental value. The bulbs were obtained from the Gansu Academy of Agricultural Sciences in Lanzhou, PR China.
Technical Methodology:
Starch and sucrose content in mother scales and bulblets were determined using iodine colorimetry and HPLC, respectively. For starch measurement, samples were extracted and mixed with an iodine solution, and the resulting complex's absorbance was measured. Sucrose was separated and quantified using HPLC. These assays were performed in three independent experiments with three biological replicates each, ensuring reliable results.
Results
The measurement of starch and sucrose concentrations revealed dynamic changes during bulblet development.
For starch, the results showed that its content in the mother scales decreased significantly during the initial 14 days of bulblet formation, indicating its utilization as an energy source for bulblet activation. Thereafter, the starch content continued to decline slowly. However, in the bulblets themselves, the starch content gradually increased throughout the entire development process, particularly during the later stages (35-70 days). This suggests that starch hydrolysis provided nutrients and energy required for bulblet growth and enlargement.
Regarding sucrose, its content exhibited a distinct pattern during bulblet development. At the beginning of bulblet formation, there was a sharp decrease in sucrose content in the mother scales, coinciding with a corresponding increase in sucrose content in the bulblets. Subsequently, although the rate of starch degradation accelerated, the sucrose content in the mother scales showed a steady decline, while in the bulblets, it continued to increase. These observations highlight the importance of sucrose as a precursor for essential metabolites and its involvement in bulblet development and growth.
Overall, the measurement of starch and sucrose concentrations provided valuable insights into the dynamic changes in carbohydrate metabolism during bulblet formation and development in lilies, indicating the mobilization and utilization of carbohydrate reserves as an essential process for successful bulb growth.
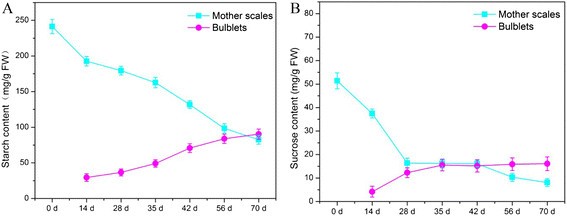 Changes of content of starch and sucrose in mother scales and bulblets of L. davidii var. unicolor.
Changes of content of starch and sucrose in mother scales and bulblets of L. davidii var. unicolor.
Reference
- Li, XueYan, et al. "Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor." BMC plant biology 14 (2014): 1-12.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Changes of content of starch and sucrose in mother scales and bulblets of L. davidii var. unicolor.
Changes of content of starch and sucrose in mother scales and bulblets of L. davidii var. unicolor.