Why Analyze Isorhamnetin?
Isorhamnetin is a bioactive flavonoid that is found in many plants, including fruits, vegetables, and medicinal herbs. Its chemical name is 3'-Methoxy-3,4',5,7-tetrahydroxyflavone, and it is commonly derived from its glycosides or from quercetin, another type of flavonoid.
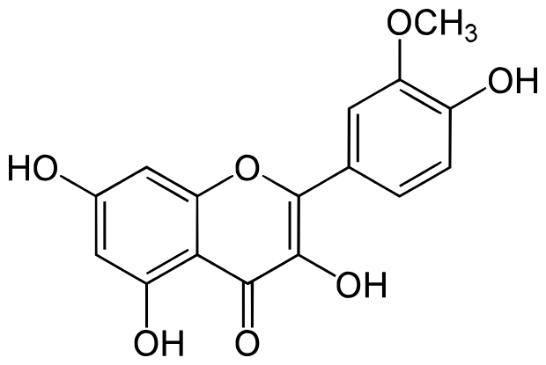 Isorhamnetin
Isorhamnetin
Isorhamnetin has gained significant attention in the scientific community due to its various biological activities. Isorhamnetin has been studied for its potential antioxidant, anti-inflammatory, antitumor, hepatoprotective, neuroprotective, and cardioprotective effects, making it an area of interest for potential therapeutic applications.
Additionally, it may have value in inhibiting adipogenesis and lipid accumulation, suggesting its potential use in managing and treating obesity.
Analyzing Isorhamnetin is essential to fully understand its properties, benefits, and potential side effects. The analysis can contribute to the development of new medicines to treat various diseases if its beneficial properties are substantiated through rigorous scientific testing. Additionally, gaining more insight could aid in developing optimal methods for its extraction and usage to ensure safety and effectiveness. In foods, it is essential to analyze it to ensure safety for consumption and to identify potential impacts on food quality and health benefits.
Specific Isorhamnetin Analysis Offered by Creative Proteomics
Creative Proteomics offers a detailed and wide-ranging analysis of Isorhamnetin. Our service covers the following project directions:
- Isorhamnetin Identification and Quantification: Using highly advanced spectrometry techniques, we provide accurate identification and quantification of isorhamnetin in various sample types.
- Interaction Studies: Investigating the interactions of isorhamnetin with other molecules and its effect on cellular pathways.
- Metabolic Profiling: Analyzing the metabolic pathways of isorhamnetin in different biological systems.
- Structural Analysis: Studying the structure of isorhamnetin and its derivatives.
- Safety Evaluation: Examining the toxicity and safety of isorhamnetin in the process of drug development.
- Bioavailability Studies: Testing the absorption, distribution, metabolism, and excretion (ADME) of isorhamnetin.
Isorhamnetin Analysis Techniques
Liquid Chromatography-Mass Spectrometry (LC-MS): LC-MS (e.g., Thermo Scientific Q Exactive™ Plus Orbitrap LC-MS/MS System and the versatile AB SCIEX Triple Quad™ 6500 LC-MS/MS System) is deemed as an excellent instrument for identifying Isorhamnetin in different matrices. LC-MS enables the separation of complex samples based on their chemical properties prior to MS detection, making it a useful tool for comprehensive metabolite profiling of Isorhamnetin.
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS (e.g., Agilent 7890B/5977A GC/MSD) occupied an unreplaceable position for volatile compound detection. For low quantities of Isorhamnetin or similar derivatives, GC-MS provides a high-resolution method that can precisely identify and quantitate Isorhamnetin amounts.
Tandem Mass Spectrometry (MS/MS): MS/MS devices such as Thermo Scientific™ Orbitrap™ provide high-resolution and high mass accuracy measurements, enabling more precise Isorhamnetin identification and quantification.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Isorhamnetin and Its Derivatives Analyzed (including but not limited to)
| Isorhamnetin |
Isorhamnetin 3-O-glucoside |
Isorhamnetin 3-O-rutinoside |
Isorhamnetin-3-O-galactoside |
| Isorhamnetin-3-O-arabinoside |
Isorhamnetin 7-O-glucoside |
Isorhamnetin 7-O-rutinoside |
Isorhamnetin 3,7-di-O-glucoside |
| Methylisorhamnetin |
|
|
|
Sample Requirements for Isorhamnetin Assay
| Sample Type |
Recommended Sample Volume |
| Plasma |
0.5-1ml |
| Serum |
0.5-1ml |
| Tissue |
50-100mg |
| Cells |
1x106 |
| Urine |
1-2ml |
Deliverables of Isorhamnetin Analysis
- A comprehensive report of the metabolite profiling with detailed data and findings.
- Raw data files and detailed procedures of the performed experiments.
- If requested, further consultation and expertise regarding the obtained results.
Case. Quercetin Metabolites in Rat Plasma Reveals Limited Conjugation Sites and Stable Metabolic Processes
Background
Understanding the metabolism of dietary flavonoids like quercetin is crucial for elucidating their potential health benefits. Previous studies have shown that quercetin metabolites in plasma are predominantly conjugated forms, but the specific types, positions of conjugation, and their quantification have not been fully characterized.
Sample
The study utilized plasma samples obtained from rats fed with quercetin glucosides over a 12-day period. These samples were subjected to LC/MS/MS analysis to identify and quantify quercetin metabolites.
Technical Platform and Procedure
Sample Preparation: Plasma samples were treated with or without deconjugation enzymes (β-glucuronidase and sulfatase) to allow for direct or deconjugated analysis, respectively.
LC/MS/MS Analysis: A comprehensive LC/MS/MS method was employed to analyze quercetin derivatives in plasma. The analysis included chromatographic separation using specific columns and detection using dynamic multiple reaction monitoring (MRM) mode.
Identification of Metabolites: The major metabolites, quercetin-7-O-glucuronide-4′-O-sulfate (QGS) and isorhamnetin-7-O-glucuronide-4′-O-sulfate (MQGS), were identified using LC-DAD/MS. Other metabolites were also detected and quantified.
Quantification: Calibration curves of each standard compound were prepared to quantify metabolites. The concentrations of quercetin derivatives in plasma were calculated from the peak area of MRM chromatograms.
Statistical Analysis: Statistical analyses were performed to assess differences among groups using ANOVA and Student's t-test or Tukey-Kramer's test.
Results
Identification of Major Metabolites: QGS and MQGS were identified as predominant quercetin derivatives in rat plasma after quercetin glucoside ingestion.
Stable Conjugation Patterns: QGS and MQGS were primarily glucuronidated at the 7-position and sulfated at the 4′-position, and their quantities remained stable over the feeding period.
Changes in Minor Metabolites: Proportions of glucuronide and diglucuronide decreased over time, while sulfation, especially quercetin-4′-O-sulfate, increased.
Direct vs. Deconjugation Analysis: Direct analysis provided comprehensive quantification of quercetin metabolites without deconjugation treatment, showing similar results to deconjugation analysis.
Implications for Human Studies: The findings have implications for human studies, suggesting that understanding the complex conjugates of quercetin in plasma could elucidate its physiological activity.
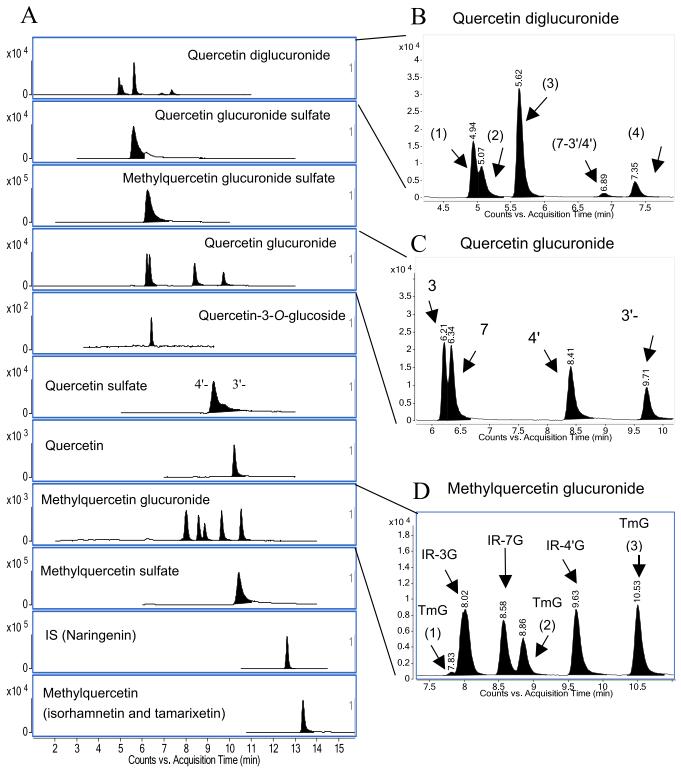 Typical MRM chromatograms of quercetin metabolites in rat plasma.
Typical MRM chromatograms of quercetin metabolites in rat plasma.
Reference
- Tanaka, Seiya, et al. "Comprehensive analyses of quercetin conjugates by LC/MS/MS revealed that isorhamnetin-7-O-glucuronide-4′-O-sulfate is a major metabolite in plasma of rats fed with quercetin glucosides." Journal of agricultural and food chemistry 67.15 (2019): 4240-4249.


 Isorhamnetin
Isorhamnetin Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Typical MRM chromatograms of quercetin metabolites in rat plasma.
Typical MRM chromatograms of quercetin metabolites in rat plasma.