What are Thioglycosides?
Thioglycosides are a class of compounds belonging to the broader category of glycosides. Glycosides are molecules composed of a sugar moiety (glycone) linked to a non-sugar component (aglycone or genin) through a glycosidic bond. In the case of thioglycosides, the glycosidic bond is formed between the sugar and a thiol group, resulting in the replacement of the typical oxygen atom with a sulfur atom.
Thioglycoside Metabolites
Thioglycosides undergo metabolic transformations in biological systems, resulting in the formation of various metabolites through enzymatic or chemical modifications. These metabolites encompass:
- Thioglycoside Sulfoxides: Generated by the oxidation of the sulfur atom in thioglycosides, leading to the introduction of a sulfoxide (-SO-) group. Sulfoxides exhibit antioxidant properties and may contribute to redox-regulatory mechanisms in biological systems.
- Thioglycoside Sulfones: Derived from further oxidation of thioglycoside sulfoxides, characterized by a sulfonyl (-SO2-) group. Sulfones potentially play roles in signaling pathways and cellular communication processes.
- Thioglycoside Sulfonates: Result from the sulfonation of thioglycosides, involving the addition of a sulfonate (-SO3-) group. Thioglycoside sulfonates are potential bioactive molecules with antibacterial and antifungal properties.
- Thioglycoside Sulfates: Formed by the sulfation of thioglycosides, introducing a sulfate (-OSO3-) group. Thioglycoside sulfates are known to participate in the regulation of cellular processes, including cell adhesion and growth.
Understanding the metabolism and actions of these thioglycoside metabolites is crucial for exploring their potential applications in drug development, glycobiology, food science, and other relevant fields. Collaborative efforts between researchers and analytical experts aim to analyze and characterize these compounds, providing valuable insights into their roles within diverse biological contexts.
Thioglycosides Analysis Service at Creative Proteomics
Metabolite Profiling and Pathway Analysis
Analyzing the diverse metabolites originating from thioglycosides is essential for comprehending the metabolic pathways and transformations these compounds undergo within biological systems. This knowledge is instrumental in unraveling the intricate relationships between thioglycosides and their downstream metabolites.
- Liquid Chromatography-Mass Spectrometry (LC-MS) represents a potent analytical technique utilized to separate and identify thioglycosides and their metabolites in complex samples. By combining liquid chromatography for effective separation and mass spectrometry for precise detection, LC-MS offers exceptional sensitivity and specificity in analyzing a wide range of thioglycoside species.
- Tandem Mass Spectrometry (MS/MS) plays a vital role in elucidating the structural information of thioglycosides and their metabolites. Through sequential ion fragmentation, MS/MS enables researchers to deduce glycosidic linkages, identify specific functional groups, and determine the precise molecular composition.
Structural Characterization of Thioglycosides
We do in-depth structural analysis of thioglycosides using cutting-edge mass spectrometry-based approaches, which includes figuring out the glycosidic bond and the shape of the sugar component.
- Electrospray ionization mass spectrometry (ESI-MS) is used to ionize thioglycosides, producing gas-phase ions that can provide valuable information about the molecular weight and ionization behavior of the compound.
- Collision-induced dissociation (CID) is used to induce fragmentation of ionized thioglycosides, which allows for the determination of sugar sequences and linkage positions within the molecule.
Quantitative Analysis of Thioglycosides
Quantifying thioglycosides is essential for obtaining precise concentration data in various biological environments, aiding in the assessment of their abundance and distribution.
- Multiple Reaction Monitoring (MRM) is a targeted quantification technique used to measure specific thioglycosides and their metabolites. MRM offers high selectivity and sensitivity, making it particularly suitable for quantifying low-abundance compounds.
- Isotope Dilution Mass Spectrometry (IDMS) is a quantitative approach that utilizes isotopically labeled internal standards to accurately determine the concentration of thioglycosides in samples. By compensating for variations in sample preparation and instrument response, IDMS enhances the accuracy of quantitative results.
Identification of Thioglycosides and Metabolites
Accurate thioglycoside identification, knowledge of the diversity of these thioglycoside-containing substances, and comprehension of possible interactions between thioglycosides and their many metabolites in complicated biological samples.
Thioglycosides Analysis Instrumentation
Triple Quadrupole Mass Spectrometer (e.g., Agilent 6460 QQQ)
Time-of-Flight Mass Spectrometer (e.g., Waters SYNAPT G2-Si)
Orbitrap Mass Spectrometer (e.g., Thermo Scientific Q Exactive HF-X)
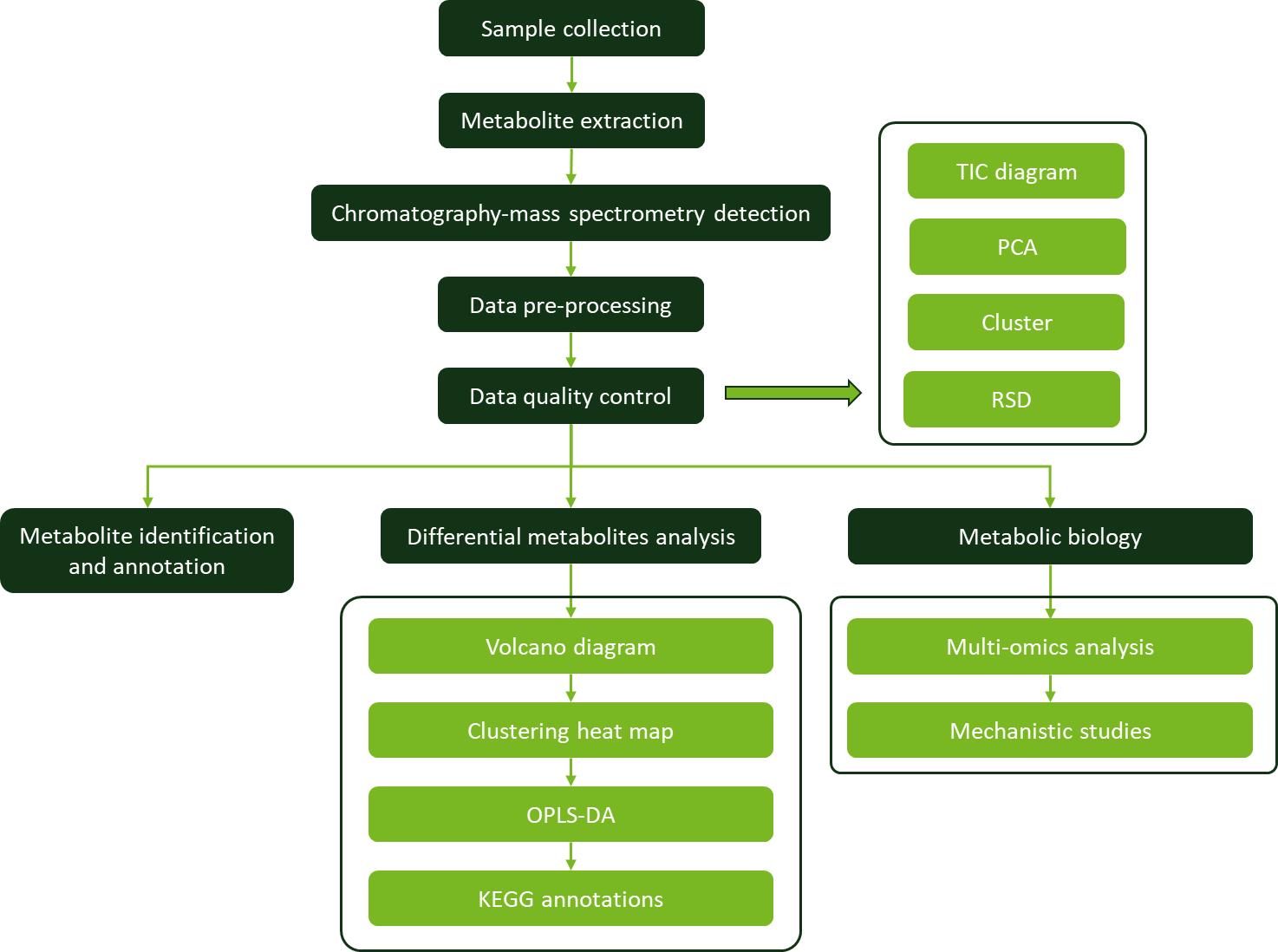 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Thioglycosides and Metabolites Analyzed (including but not limited to)
| Sugar Moiety |
Thioglycosides |
Metabolites |
| Glucose |
Glucothioglycoside |
Glucothioglycoside Sulfoxide |
| Glucothioglycoside Sulfone |
| Glucothioglycoside Sulfate |
| Glucothioglycoside Sulfonate |
|
| Glucosaminylthioglycoside |
Glucosaminylthioglycoside Sulfoxide |
| Glucosaminylthioglycoside Sulfone |
| Glucosaminylthioglycoside Sulfate |
| Glucosaminylthioglycoside Sulfonate |
| Galactose |
Galactothioglycoside |
Galactothioglycoside Sulfoxide |
| Galactothioglycoside Sulfone |
| Galactothioglycoside Sulfate |
| Galactothioglycoside Sulfonate |
| Mannose |
Mannothioglycoside |
Mannothioglycoside Sulfoxide |
| Mannothioglycoside Sulfone |
| Mannothioglycoside Sulfate |
| Mannothioglycoside Sulfonate |
| Fucose |
Fucothioglycoside |
Fucothioglycoside Sulfoxide |
| Fucothioglycoside Sulfone |
| Fucothioglycoside Sulfate |
| Fucothioglycoside Sulfonate |
| N-Acetylglucosamine |
N-Acetylglucosaminylthioglycoside |
N-Acetylglucosaminylthioglycoside Sulfoxide |
| N-Acetylglucosaminylthioglycoside Sulfone |
| N-Acetylglucosaminylthioglycoside Sulfate |
| N-Acetylglucosaminylthioglycoside Sulfonate |
| N-Acetylgalactosamine |
N-Acetylgalactosaminylthioglycoside |
N-Acetylgalactosaminylthioglycoside Sulfoxide |
| N-Acetylgalactosaminylthioglycoside Sulfone |
| N-Acetylgalactosaminylthioglycoside Sulfate |
| N-Acetylgalactosaminylthioglycoside Sulfonate |
| Sialic Acid |
Sialothioglycoside |
Sialothioglycoside Sulfoxide |
| Sialothioglycoside Sulfone |
| Sialothioglycoside Sulfate |
| Sialothioglycoside Sulfonate |
Sample Requirements for Thioglycosides Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
50 mg - 1 g |
| Animal Samples |
Tissues |
100 mg - 1 g |
| Cell Samples |
Cells and Culture |
106 - 108 cells |
Case 1. Discovery of New Thioglycosides from Wasabia japonica Root with Potential Biological Activities
Background:
Thioglycosides are a rare class of compounds found in Nature, primarily reported from plants in the family Brassicaceae, certain microorganisms, and a marine sponge, Clathria pyramida. Among these compounds, glucosinolate derivatives featuring a unique Osulfated thiohydroximate of 1-thio-β-D-glucopyranose are the major class found in plants. Other types of thioglycosides have also been reported. Wasabia japonica, commonly known as wasabi, belongs to the family Brassicaceae and has been widely used as a pungent spice for sushi and sashimi. Since desulfosinigrin is the only known thioglycoside identified from wasabi, the study aimed to search for other thioglycosides with potential biological activities.
Samples:
The roots of Wasabia japonica were used as the sample for this study. A total of six new thioglycosides (1-6) and one known analogue (7) were isolated from the roots of W. japonica.
Methods:
Extraction and Isolation: The roots of W. japonica were extracted and partitioned to obtain EtOAc-soluble and n-BuOH-soluble fractions. The EtOAc-soluble fraction was further separated into six fractions (E1-E6) using Diaion HP-20 resin. The n-BuOH-soluble fraction was separated into ten fractions (B1-B10). The compounds of interest were isolated through chromatography techniques such as silica gel column chromatography and Lobar-A RP-C18 column chromatography. Semipreparative HPLC was also employed to obtain pure compounds.
Characterization: The isolated compounds were characterized using various spectroscopic techniques. The 1H and 13C NMR data were obtained in different solvents, and the molecular formulas were determined using HRESIMS.
Computational Analysis: Computational analysis was performed to evaluate the conformational states of compound 1. The Gaussian 09 package was used for calculations, and the coupling constants and GIAO shielding constants were determined to aid in the structural elucidation.
Bioactivity Testing: The isolated compounds were tested for their potential anti-inflammatory activity by measuring nitric oxide (NO) production levels in LPS-stimulated murine microglia. Some of the compounds were also tested for their anti-inflammatory, neuroprotective, and cytotoxic activities.
Results
From the roots of W. japonica, six new thioglycosides (1-6) and one known analogue (7) were identified. Among these, compounds 1-3 were rare derivatives possessing a disulfide bond connecting the carbohydrate motif and the aglycone. Compound 1, termed wasulfiside A, exhibited moderate anti-inflammatory activity. The structures of these compounds were elucidated through NMR data analyses, HRMS, and computational methods (DP4 and CP3). Additionally, the absolute configurations of the compounds were determined using various spectroscopic techniques.
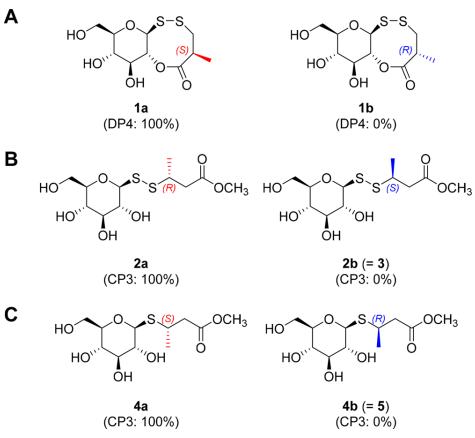 DP4 and CP3 analysis results: (A) 1, (B) 2/3, (C) 4/5.
DP4 and CP3 analysis results: (A) 1, (B) 2/3, (C) 4/5.
Reference
- Kim, Chung Sub, et al. "Rare thioglycosides from the roots of Wa sabia japonica." Journal of natural products 81.9 (2018): 2129-2133.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service DP4 and CP3 analysis results: (A) 1, (B) 2/3, (C) 4/5.
DP4 and CP3 analysis results: (A) 1, (B) 2/3, (C) 4/5.