Why Analyze Rhamnazin?
Rhamnazin is a naturally occurring flavonoid, a type of plant secondary metabolite known for its wide range of biological and pharmacological activities. Structurally, it is a methylated derivative of quercetin, one of the most abundant flavonoids, which contributes to the antioxidant capacity of foods like fruits and vegetables. Rhamnazin distinguishes itself through its unique chemical structure, featuring methoxy groups that confer specific bioactivities not found in its parent compound, quercetin. This structural variation enhances its solubility, stability, and potential for bioactivity, making rhamnazin a compound of interest in nutritional science, pharmacology, and therapeutic applications.
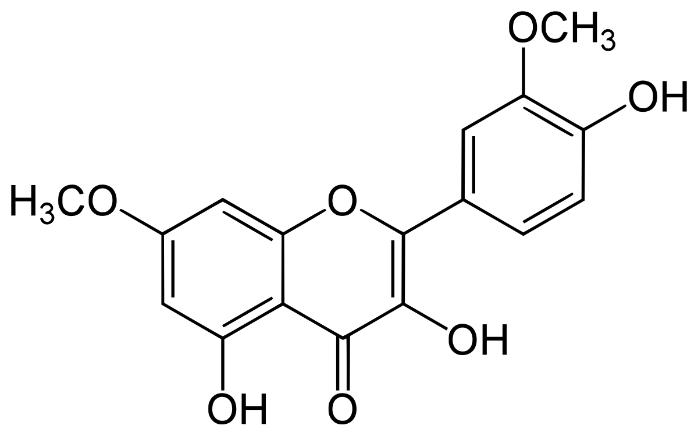 Rhamnazin
Rhamnazin
Rhamnazin exhibits a broad spectrum of biological activities, including antioxidant, anti-inflammatory, and anti-cancer properties. These activities are largely attributed to its ability to scavenge reactive oxygen species (ROS), thereby reducing oxidative stress within the body. Oxidative stress is a known contributor to various chronic diseases, such as cancer, heart disease, and neurodegenerative disorders. Therefore, rhamnazin's antioxidant capacity could potentially offer protective effects against these conditions.
Moreover, rhamnazin has been shown to modulate several key signaling pathways involved in inflammation and cell proliferation. By inhibiting specific enzymes and interacting with cellular receptors, it can exert anti-inflammatory effects and inhibit the growth of certain cancer cells. These attributes underscore the therapeutic potential of rhamnazin in managing and potentially treating various health conditions.
Creative Proteomics can provide you with rhamnazin analysis for a variety of needs.
Specific Rhamnazin Analysis Offered by Creative Proteomics
Purity and Composition Analysis
- High-Purity Rhamnazin Analysis: We provide accurate quantification of Rhamnazin purity, essential for research and commercial applications where high-purity compounds are required.
- Impurity Profile Identification: Our services include the identification and quantification of impurities that may be present in Rhamnazin samples, aiding in the assessment of sample quality and purity.
Metabolite Profiling and Bioavailability Studies
- Metabolite Identification: Through advanced mass spectrometry techniques, we identify and characterize Rhamnazin metabolites, providing insights into its metabolic pathways.
- Bioavailability Assessment: We evaluate the bioavailability of Rhamnazin, which is crucial for understanding its efficacy and potential therapeutic applications.
Pharmacodynamics and Mechanism of Action
- Activity Screening: Our services include screening for biological activity of Rhamnazin and its metabolites, helping to elucidate its pharmacological properties.
- Mechanism of Action (MoA) Studies: We employ a range of analytical techniques to investigate the molecular mechanisms through which Rhamnazin exerts its biological effects.
Safety and Toxicology Assessment
- Toxicological Screening: We offer comprehensive toxicological screening services to assess the safety profile of Rhamnazin, crucial for its development as a therapeutic agent.
- ADME (Absorption, Distribution, Metabolism, and Excretion) Studies: Our ADME studies provide valuable information on the pharmacokinetics of Rhamnazin, informing safety and efficacy evaluations.
Quality Control for Dietary Supplements and Food Products
- Content Verification: We verify the labeled content of Rhamnazin in dietary supplements and food products, ensuring compliance with regulatory standards and consumer safety.
- Stability Testing: Our services include stability testing of Rhamnazin under various conditions, essential for product formulation and shelf-life determination.
Rhamnazin Analysis Techniques
At Creative Proteomics, we harness the power of state-of-the-art mass spectrometry (MS) technology to deliver precise and accurate rhamnazin analysis. Our technical platform includes:
- High-Resolution Mass Spectrometry (HRMS): For detailed molecular characterization and quantification of rhamnazin, we utilize the Thermo Fisher Scientific™ Orbitrap™ series (e.g., Orbitrap Fusion™ Lumos™ Tribrid™ Mass Spectrometer), renowned for its exceptional resolution, accuracy, and sensitivity.
- Liquid Chromatography-Mass Spectrometry (LC-MS): The Agilent 1290 Infinity II LC system coupled with the Agilent 6546 LC/Q-TOF MS provides unparalleled analytical performance for the separation and detection of rhamnazin and its metabolites.
- Tandem Mass Spectrometry (MS/MS): For targeted analysis and structural elucidation of rhamnazin, we employ the Sciex Triple Quad™ 6500+ system, offering superior sensitivity and specificity.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Rhamnazin Assay
| Sample Type |
Sample Amount Required |
| Serum/Plasma |
500 µL |
| Blood |
500 µL |
| Urine |
1 mL |
| Saliva |
1 mL |
| Tissue |
50 mg |
| Skin Biopsy |
10 mg |
| Plant Extract |
100 mg |
| Herbal Preparation |
200 mg |
| Plant Material |
1 g |
| Essential Oil |
100 µL |
| Food Product |
10 g |
Deliverables of Rhamnazin Analysis
- Analysis Report: A detailed report summarizing methodologies, experimental procedures, and results.
- Quantification Data: Accurate quantification of rhamnazin and related compounds.
- Metabolite Profiling: Identification of rhamnazin metabolites, if applicable.
- Mechanistic Insights: Understanding of rhamnazin's biological effects and mode of action.
- Recommendations: Tailored suggestions for further research or experimentation.
Applications of Rhamnazin Analysis
Pharmaceutical Research and Development
In the pharmaceutical sector, rhamnazin analysis is pivotal for drug discovery and development processes. By understanding the compound's pharmacokinetics and pharmacodynamics, researchers can design more effective therapeutic agents with targeted delivery mechanisms. Analysis of rhamnazin aids in:
- Identifying Active Metabolites: Determining which metabolites of rhamnazin possess bioactivity and contribute to its therapeutic effects.
- Optimizing Formulations: Enhancing the stability and bioavailability of rhamnazin in pharmaceutical formulations.
- Toxicology Assessments: Evaluating the safety profile of rhamnazin through detailed toxicological analysis.
Nutritional Science and Functional Foods
Rhamnazin analysis is crucial for the development of functional foods and dietary supplements aimed at improving health and preventing diseases.
- Bioavailability Studies: Investigating how different food matrices and processing methods affect the absorption and efficacy of rhamnazin.
- Efficacy Validation: Supporting health claims of functional foods and supplements by quantifying the rhamnazin content and its bioactivity.
- Nutrient-Nutrient Interaction Studies: Understanding how rhamnazin interacts with other nutrients, which can influence its effectiveness when consumed as part of a complex diet.
Quality Control and Safety Assurance
Ensuring the quality and safety of rhamnazin-containing products is essential for consumer protection and regulatory compliance.
- Content Verification: Confirming that products contain the amount of rhamnazin declared on the label.
- Detecting Adulterants and Contaminants: Identifying potential impurities or contaminants that could compromise product safety.
- Shelf-Life Testing: Assessing the stability of rhamnazin in products over time to determine expiration dates and optimal storage conditions.
Environmental Monitoring
Rhamnazin and its derivatives can also be markers of environmental exposure and pollutants.
- Biomonitoring: Detecting and quantifying rhamnazin in biological samples as an indicator of exposure to certain plants or environmental contaminants.
- Ecotoxicology Studies: Understanding the impact of rhamnazin and related compounds on wildlife and ecosystems, particularly in areas near agricultural activities where flavonoids might be used as natural pesticides or herbicides.
Academic and Clinical Research
In academia and clinical settings, rhamnazin analysis facilitates:
- Mechanistic Studies: Elucidating the cellular and molecular mechanisms by which rhamnazin exerts its health effects.
- Clinical Trials: Monitoring rhamnazin levels in participants to correlate its pharmacokinetics with pharmacodynamics outcomes, aiding in the determination of therapeutic doses and schedules.
Case. Electrochemical Characterization and Oxidation Pathways of Rhamnazin in Aqueous Solution
Background
Rhamnazin, a natural compound found in various plants, exhibits potential pharmacological properties, including antioxidant activity. Understanding its electrochemical behavior is crucial for elucidating its reactivity and potential applications.
Sample
Rhamnazin (compound 1) was studied using cyclic voltammetry on a glassy carbon electrode in buffered aqueous solutions at varying pH levels.
Technical Platform and Procedure
- Cyclic Voltammetry: Used to analyze the oxidation behavior of rhamnazin, revealing three oxidation waves corresponding to distinct molecular transformations.
- UV-Vis Spectroelectrochemistry: Employed to monitor spectral changes during electrolysis, aiding in the identification of oxidation products such as 2,4-dihydroxy-2-(4-hydroxy-3-methoxybenzoyl)-6-methoxy-benzofuran-3(2H)-one (R1).
- HPLC-DAD and HPLC-ESI-MS: Utilized for further characterization of oxidation products, confirming the presence of R1 and its subsequent decomposition under ambient conditions.
Results
Electrochemical Behavior: Three oxidation waves were observed for rhamnazin, with the first wave corresponding to the oxidation of a hydroxyl group at ring B. pH dependence was evident, with peak potentials shifting with pH variations. Compound 2 exhibited different electrochemical behavior in alkaline solutions, suggesting structural influences on oxidation processes.
Spectroelectrochemical Analysis: UV-Vis spectra revealed characteristic changes during electrolysis, indicating the formation of oxidation products. The main product, identified as R1, underwent subsequent chemical reactions, leading to its decomposition at higher potentials.
Oxidation Under Ambient Conditions: Rhamnazin exhibited rapid degradation in the presence of atmospheric oxygen, forming unstable oxidation products. HPLC-DAD chromatograms confirmed the presence of these products, which were further characterized using ESI-Q-ToF spectra.
Conclusion: The oxidation potential of rhamnazin was found to be pH-dependent, involving a two-electron process. Two oxidation pathways were identified, with one favored in the absence of oxygen. The instability of rhamnazin under ambient conditions highlights the challenges in its handling and underscores the importance of careful experimental design for reliable data acquisition.
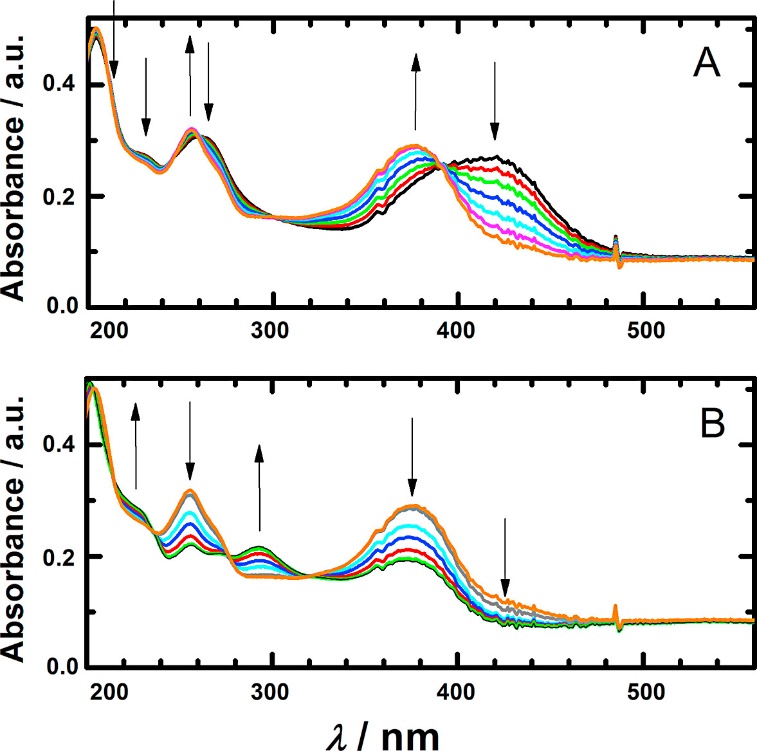 The in situ spectroelectrochemistry of 6 × 10−4 mol l−1 rhamnazin in solution of Britton Robinson buffer and 40% ethanol (v/v), pH = 9.0, (A) at the potential behind the oxidation wave I, and (B) at the potential of the oxidation wave II.
The in situ spectroelectrochemistry of 6 × 10−4 mol l−1 rhamnazin in solution of Britton Robinson buffer and 40% ethanol (v/v), pH = 9.0, (A) at the potential behind the oxidation wave I, and (B) at the potential of the oxidation wave II.
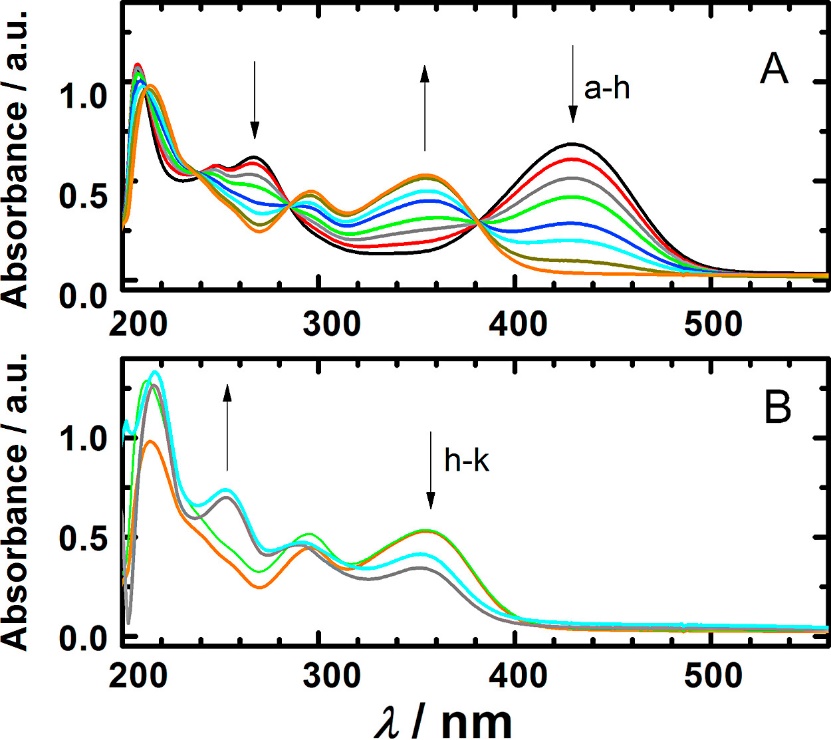 Absorption spectra of 3.6 × 10−5 mol l−1 rhamnazin in 0.1 mol l−1 KCl and 3.6 × 10−3 mol l−1 KOH (A) during exposure to atmospheric oxygen for (a) 0, (b) 360, (c) 560, (d) 760, (e) 1160, (f) 1560, (g) 2520, (h) 4520 s, and (B) (h) 4520 s, (i) 92 min, (j) 122 h and (k) 124 h.
Absorption spectra of 3.6 × 10−5 mol l−1 rhamnazin in 0.1 mol l−1 KCl and 3.6 × 10−3 mol l−1 KOH (A) during exposure to atmospheric oxygen for (a) 0, (b) 360, (c) 560, (d) 760, (e) 1160, (f) 1560, (g) 2520, (h) 4520 s, and (B) (h) 4520 s, (i) 92 min, (j) 122 h and (k) 124 h.
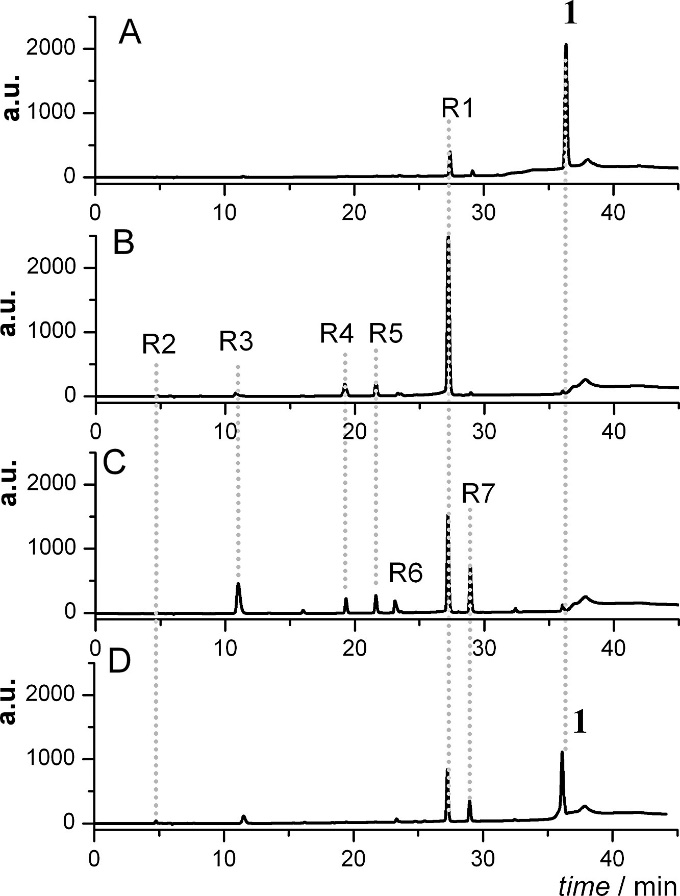 HPLC-DAD chromatogram of a solution of 1 in the absence of oxygen (A) before the electrolysis and (B) after the electrolysis. (C) and (D): chromatograms of solutions collected during and before electrolysis and exposed to the air oxygen, respectively.
HPLC-DAD chromatogram of a solution of 1 in the absence of oxygen (A) before the electrolysis and (B) after the electrolysis. (C) and (D): chromatograms of solutions collected during and before electrolysis and exposed to the air oxygen, respectively.
Reference
- Ramešová, Šárka, Ilaria Degano, and Romana Sokolová. "Two oxidation pathways of bioactive flavonol rhamnazin under ambient conditions." Electrochimica Acta 133 (2014): 359-363.


 Rhamnazin
Rhamnazin Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service The in situ spectroelectrochemistry of 6 × 10−4 mol l−1 rhamnazin in solution of Britton Robinson buffer and 40% ethanol (v/v), pH = 9.0, (A) at the potential behind the oxidation wave I, and (B) at the potential of the oxidation wave II.
The in situ spectroelectrochemistry of 6 × 10−4 mol l−1 rhamnazin in solution of Britton Robinson buffer and 40% ethanol (v/v), pH = 9.0, (A) at the potential behind the oxidation wave I, and (B) at the potential of the oxidation wave II. Absorption spectra of 3.6 × 10−5 mol l−1 rhamnazin in 0.1 mol l−1 KCl and 3.6 × 10−3 mol l−1 KOH (A) during exposure to atmospheric oxygen for (a) 0, (b) 360, (c) 560, (d) 760, (e) 1160, (f) 1560, (g) 2520, (h) 4520 s, and (B) (h) 4520 s, (i) 92 min, (j) 122 h and (k) 124 h.
Absorption spectra of 3.6 × 10−5 mol l−1 rhamnazin in 0.1 mol l−1 KCl and 3.6 × 10−3 mol l−1 KOH (A) during exposure to atmospheric oxygen for (a) 0, (b) 360, (c) 560, (d) 760, (e) 1160, (f) 1560, (g) 2520, (h) 4520 s, and (B) (h) 4520 s, (i) 92 min, (j) 122 h and (k) 124 h. HPLC-DAD chromatogram of a solution of 1 in the absence of oxygen (A) before the electrolysis and (B) after the electrolysis. (C) and (D): chromatograms of solutions collected during and before electrolysis and exposed to the air oxygen, respectively.
HPLC-DAD chromatogram of a solution of 1 in the absence of oxygen (A) before the electrolysis and (B) after the electrolysis. (C) and (D): chromatograms of solutions collected during and before electrolysis and exposed to the air oxygen, respectively.