Why Analyze Flavonoids?
Flavonoids are a diverse group of plant compounds known for their polyphenolic structure and various biological activities. They play essential roles in plant physiology, defense mechanisms, and interactions with the environment. Different subclasses of flavonoids exist, each with distinct chemical structures and functions.
Flavonoids have significant contributions to plant growth and development. They participate in processes such as pigmentation, UV protection, defense against pathogens and pests, and symbiotic relationships with other organisms. Studying flavonoid metabolism provides valuable insights into their biosynthesis pathways, regulation, and roles in plants. This knowledge can be applied in breeding and metabolic engineering programs to enhance flavonoid content or modify their profiles to achieve desired traits, such as improved nutritional value or increased environmental resilience.
Flavonoids also play crucial roles in plant-environment interactions and ecological processes. They are involved in plant-pollinator relationships, attracting pollinators and facilitating plant reproduction. Flavonoids can also influence neighboring plants and organisms through allelopathic reactions, impacting plant community dynamics and biodiversity. Analyzing flavonoid metabolism and their ecological functions enhances our understanding of ecosystem dynamics and conservation efforts.
Moreover, flavonoids have gained attention for their potential health benefits. They exhibit antioxidant properties, protecting cells from oxidative damage caused by free radicals. Flavonoids also possess anti-inflammatory properties, contributing to the reduction of chronic inflammation. Some flavonoids show anticancer effects, cardiovascular protection, and immune system support. Investigating flavonoids allows researchers to uncover their mechanisms of action and explore their potential applications in disease prevention.
Flavonoids Analysis at Creative Proteomics
1. Flavonoid Identification:
Creative Proteomics employs state-of-the-art mass spectrometry techniques coupled with chromatographic separation to accurately identify and characterize various flavonoid compounds present in complex biological samples. This analysis enables the determination of molecular weight, fragmentation patterns, and structural elucidation of flavonoids.
2. Flavonoid Profiling:
The flavonoid profiling service at Creative Proteomics offers a comprehensive analysis of the flavonoid composition in different samples. Using advanced chromatographic techniques, such as high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC), combined with mass spectrometry, this analysis provides a detailed quantification and identification of individual flavonoid compounds present in a given sample.
3. Quantitative Analysis of Flavonoids:
Creative Proteomics provides precise quantification of flavonoids using validated analytical methods. This analysis utilizes stable isotope-labeled internal standards and advanced LC-MS/MS techniques to accurately determine the concentration of specific flavonoids in various matrices, including plant extracts, food products, and biological fluids.
4. Flavonoid Metabolism Studies:
Creative Proteomics offers metabolomic analysis to investigate the metabolic pathways and transformations of flavonoids in biological systems. By employing high-resolution mass spectrometry and bioinformatics approaches, this analysis provides valuable insights into the metabolism of flavonoids, their metabolites, and their interactions with cellular pathways.
5. Flavonoid Bioactivity Screening:
Creative Proteomics performs bioactivity screening of flavonoids to evaluate their potential health benefits and biological activities. This analysis involves various in vitro and in vivo assays to assess antioxidant, anti-inflammatory, anticancer, and other bioactive properties of flavonoids, enabling the identification of compounds with therapeutic potential.
Flavonoid Metabolism Analysis Techniques
Flavonoid metabolism analysis relies on various mass spectrometry-based techniques, which offer high sensitivity, specificity, and versatility. Some of the commonly employed techniques include:
- LC-MS combines liquid chromatography, which separates complex mixtures of metabolites, with mass spectrometry, which detects and quantifies individual compounds based on mass-to-charge ratio. Instruments such as the Agilent 6495 Triple Quadrupole LC-MS/MS and Thermo Scientific Q Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer are commonly used for flavonoid analysis.
- GC-MS involves the separation of volatile metabolites by gas chromatography and subsequent detection and identification by mass spectrometry. It is particularly useful for analyzing volatile flavonoid derivatives. Instruments such as the Agilent 7890B GC system coupled with the Agilent 5977B GC/MSD system are commonly employed for this purpose.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Flavonoids Analyzed (including but not limited to)
| Types |
Compounds |
| Flavones |
Apigenin, Luteolin, Tangeretin, Chrysin, Baicalein, Diosmetin, Wogonin, Scutellarein, Genkwanin |
| Flavanols |
Catechin, Epicatechin, Epicatechin gallate, Epigallocatechin, Epigallocatechin gallate, Gallocatechin, Procyanidins |
| Flavonols |
Quercetin, Kaempferol, Myricetin, Isorhamnetin, Fisetin, Galangin, Rutin, Morin, Hesperetin, Taxifolin |
| Flavanones |
Naringenin, Hesperidin, Eriodictyol, Naringin, Eriocitrin, Neohesperidin, Liquiritin, Poncirin, Isosakuranetin |
| Flavanonols |
Eriodictyol, Taxifolin, Pinobanksin, Sakuranetin, Naringenin, Hesperetin, Fustin, Isosakuranetin, Homoeriodictyol, Pinocembrin, Tricetin, Chrysin, Galangin, Apigenin, Eupatorin, Myricetin, Kaempferol, Morin, Rhamnetin, Quercetin, Fisetin, Luteolin, Diosmetin, Tangeretin, Luteolin-7-glucoside, Naringin, Hesperidin, Rutin, Narirutin, Poncirin. |
| Dihydroflavonols |
Dihydroquercetin, Dihydrokaempferol, Dihydromyricetin, Dihydroisorhamnetin, Dihydrofisetin, Dihydrogalangin, Dihydrorutin, Dihydromorin |
| Dihydroflavones |
Dihydroapigenin, Dihydroluteolin, Dihydrotangeretin, Dihydrochrysin, Dihydrobaicalein, Dihydrodiosmetin, Dihydrowogonin, Dihydrosctuellarein |
| Anthocyanidins |
Cyanidin, Delphinidin, Malvidin, Pelargonidin, Petunidin, Peonidin, Rosinidin, Chrysanthemin |
| Flavonoid Glycosides |
Quercitrin, Kaempferol-3-O-rutinoside, Rutinose, Quercetin-3-O-glucoside, Isorhamnetin-3-O-rutinoside, Naringenin-7-O-glucoside, Taxifolin |
| Isoflavones |
Genistein, Daidzein, Glycitein, Glycitin, Biochanin A, Formononetin, Prunetin, Calycosin, Puerarin |
| Chalcones |
Dihydrochalcone, Phloretin, Xanthohumol, Isoliquiritigenin, Butin, Butein, Liquiritigenin, Chalconaringenin |
| Neoflavonoids |
Quercetin, Kaempferol, Myricetin, Naringenin, Isorhamnetin, Apigenin, Kaempferide, Rhamnazin, Taxifolin, Abyssinone, Amentoflavone, Engeletin, Eupatilin, Isosophoraflavone, Pterocarpan, Sophoraflavone, Pterocarpin, Podocarpusflavone A, Sandwicensin, Trihydroxyflavone, Pinocembrin |
Why Choose Us?
- Detection platform: Untargeted metabolome+targeted metabolome detection technology for metabolite detection, taking into account both qualitative and quantitative accuracy.
- Comprehensive database: Self-built metabolite database for plant species, with 3700+ substances in the database.
- Substance richness: Full coverage of flavonoids, flavonols, flavanones, dihydroflavonols, dihydroflavonoids, anthocyanins, flavonoid glycosides, isoflavonoids, chalcones and other flavonoids.
- Stable detection: 6 quality controls to provide high quality data.
- Leading in the number of substances detected: 200-500 metabolites detected in a single run.
Sample Requirements for Flavonoids Metabolism Assay
| Sample Types |
Minimum Sample Size |
Biological Repeat |
| Plant Samples |
Stem, bud, node, leaf, flower, fruit, root, healing tissue |
600mg |
3-6 |
| Liquid Samples |
Root secretions |
10mL |
| Fermentation fluid, wine, tissue fluid, fruit juice |
5mL |
| Honey, nectar, oil, extracts |
500uL |
| Specialty Samples |
Cultured samples, presence of liquids |
600mg |
Case 1. Antioxidant Potential of Ephedra alata: Phenolic Compounds Analysis
Background:
Phenolic compounds and flavonoids are secondary metabolites found in plants and are known for their potential health benefits and antioxidant properties. These compounds have been studied extensively due to their role in disease resistance and protection against oxidative stress. Analyzing the phenolic and flavonoid content in plant extracts provides valuable information about their antioxidant potential and bioactive components.
Sample:
The sample used in this study was Ephedra alata, a medicinal plant collected from the southern part of the West Bank, Palestine. The plant material was properly authenticated and underwent a drying process before extraction. The dried plant material was ground into a powdered form for further analysis.
Technical Platform and Procedure:
The extraction of phenolic and flavonoid compounds was performed using different solvents: water, 80% ethanol, and 100% ethanol. The extraction process involved subjecting the plant material to sonication-assisted extraction at 37°C for three hours. The extracts were then filtered, and the filtrate was evaporated using a rotary evaporator. The resulting crude extracts were stored for subsequent analysis.
Quantification and Identification:
Total phenolic content (TPC) and total flavonoid content (TFC) of the extracts were determined using colorimetric assays. Folin-Ciocalteu and aluminum chloride colorimetric methods were employed for TPC and TFC evaluation, respectively. The antioxidant activity (AA) of the extracts was assessed using different assays such as ferric reducing/antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), and DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay.
To identify and analyze the phenolic compounds present in the extracts, high-performance liquid chromatography (HPLC) with photodiode array (PDA) detection and liquid chromatography-mass spectrometry (LC-MS) techniques were utilized. Chromatographic conditions such as column type, mobile phase composition, and detection wavelengths were optimized to achieve efficient separation and detection of the compounds.
Results
The study demonstrated that the choice of extraction solvent significantly influenced the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (AA) of the Ephedra alata extracts. Extracts obtained using 80% ethanol exhibited the highest TPC and AA values, followed by those obtained with 100% ethanol and water. Conversely, TFC was found to be highest in the order of 100% ethanol, 80% ethanol, and water.
Through simultaneous HPLC-PDA and LC-MS analysis, specific phenolic compounds were identified in the ethanolic extracts of Ephedra alata, including Luteolin-7-O-glucuronide flavone and Myricetin 3-rhamnoside. These compounds were characterized based on their unique UV-Vis spectra and mass spectrometry data.
In summary, the study highlighted the abundant presence of potent flavonoid glycosidic compounds in Ephedra alata extracts, as supported by the analytical findings. The presence of these compounds suggests that Ephedra alata could be a valuable natural source of potent antioxidants, with potential applications in various industries such as food, cosmetics, and pharmaceuticals.
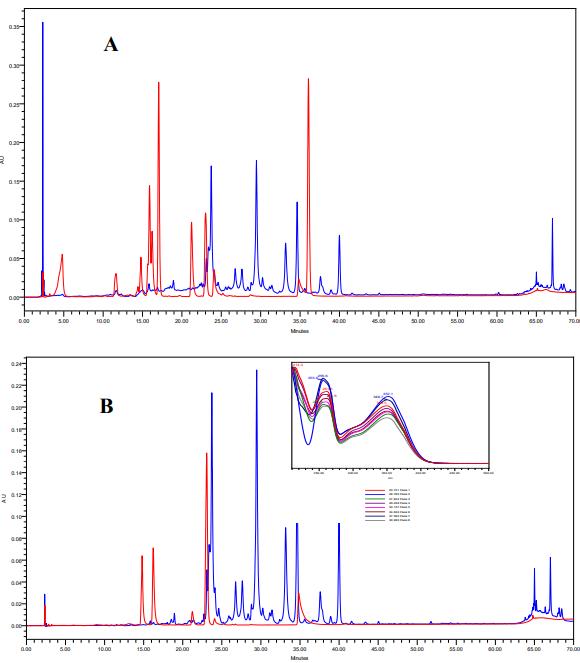 Overlaid chromatograms of phenolic and flavonoids standards mixture and 100% ethanol extract of Ephera alata at 272 nm (A) and 350 nm (B) respectively
Overlaid chromatograms of phenolic and flavonoids standards mixture and 100% ethanol extract of Ephera alata at 272 nm (A) and 350 nm (B) respectively
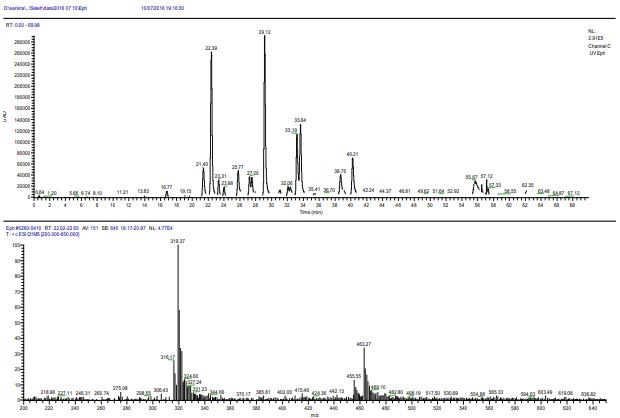 UHPLC of the Ephedra alata ethanol extract (A) and the (+)-ESI mass spectrum of Luteolin-7-Oglucuronide flavonoid.
UHPLC of the Ephedra alata ethanol extract (A) and the (+)-ESI mass spectrum of Luteolin-7-Oglucuronide flavonoid.
Reference
- Al-Rimawi, Fuad, et al. "Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity." African Journal of Traditional, Complementary and alternative medicines 14.2 (2017): 130-141.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Overlaid chromatograms of phenolic and flavonoids standards mixture and 100% ethanol extract of Ephera alata at 272 nm (A) and 350 nm (B) respectively
Overlaid chromatograms of phenolic and flavonoids standards mixture and 100% ethanol extract of Ephera alata at 272 nm (A) and 350 nm (B) respectively UHPLC of the Ephedra alata ethanol extract (A) and the (+)-ESI mass spectrum of Luteolin-7-Oglucuronide flavonoid.
UHPLC of the Ephedra alata ethanol extract (A) and the (+)-ESI mass spectrum of Luteolin-7-Oglucuronide flavonoid.