What is Myricetin?
Myricetin is a naturally occurring bioflavonoid found in various fruits, vegetables, herbs, and beverages such as tea and wine. It possesses anti-oxidative, anti-inflammatory, and anti-cancer benefits, thus playing a pivotal role in preventive healthcare. To fully understand and harness these pharmacological effects, accurate analysis of myricetin is vital.
Creative Proteomics provides an extensive, precise, and reliable myricetin analysis service. The identification and quantification of myricetin and its derivatives aid in evaluating their therapeutic potential and metabolism in the body. Additionally, this analysis provides important insights into the biochemical, physiological, and pathological roles of myricetin in human health and disease.
 Myricetin
Myricetin
Specific Myricetin Analysis Offered by Creative Proteomics
- Myricetin Identification: Identifying its presence within complex biological samples.
- Quantitative Analysis: Estimating the concentration of myricetin within provided samples.
- Bioactivity evaluation of Myricetin: Studying its various biological effects.
- Structural Elucidation: Determining the structural components of myricetin.
- Metabolic Profiling: Monitoring changes in myricetin metabolite levels as a function of nutrition, disease, drug treatment, or genetic modification.
Myricetin Analysis Techniques
Advancements in analytical techniques such as Mass Spectrometry have allowed the wide-ranging study of myricetin. At Creative Proteomics, we employ cutting-edge technologies, like Liquid Chromatography-Mass Spectrometry (LC-MS), and Gas Chromatography-Mass Spectrometry (GC-MS).
Liquid Chromatography-Mass Spectrometry (LC-MS): Ideal for analyzing heat-sensitive and non-volatile samples, LC-MS is extensively used for the detection of myricetin. The use of LC-MS/MS (Tandem Mass Spectrometry) using QTRAP system extends the sensitivity and provides data on structural identity.
Gas Chromatography-Mass Spectrometry (GC-MS): Hailed for its high efficiency and resolution, GC-MS is suitable for volatile samples. Thermo Fisher's Q Exactive GC Orbitrap GC-MS/MS system is available in our labs for delivering accurate myricetin analysis.
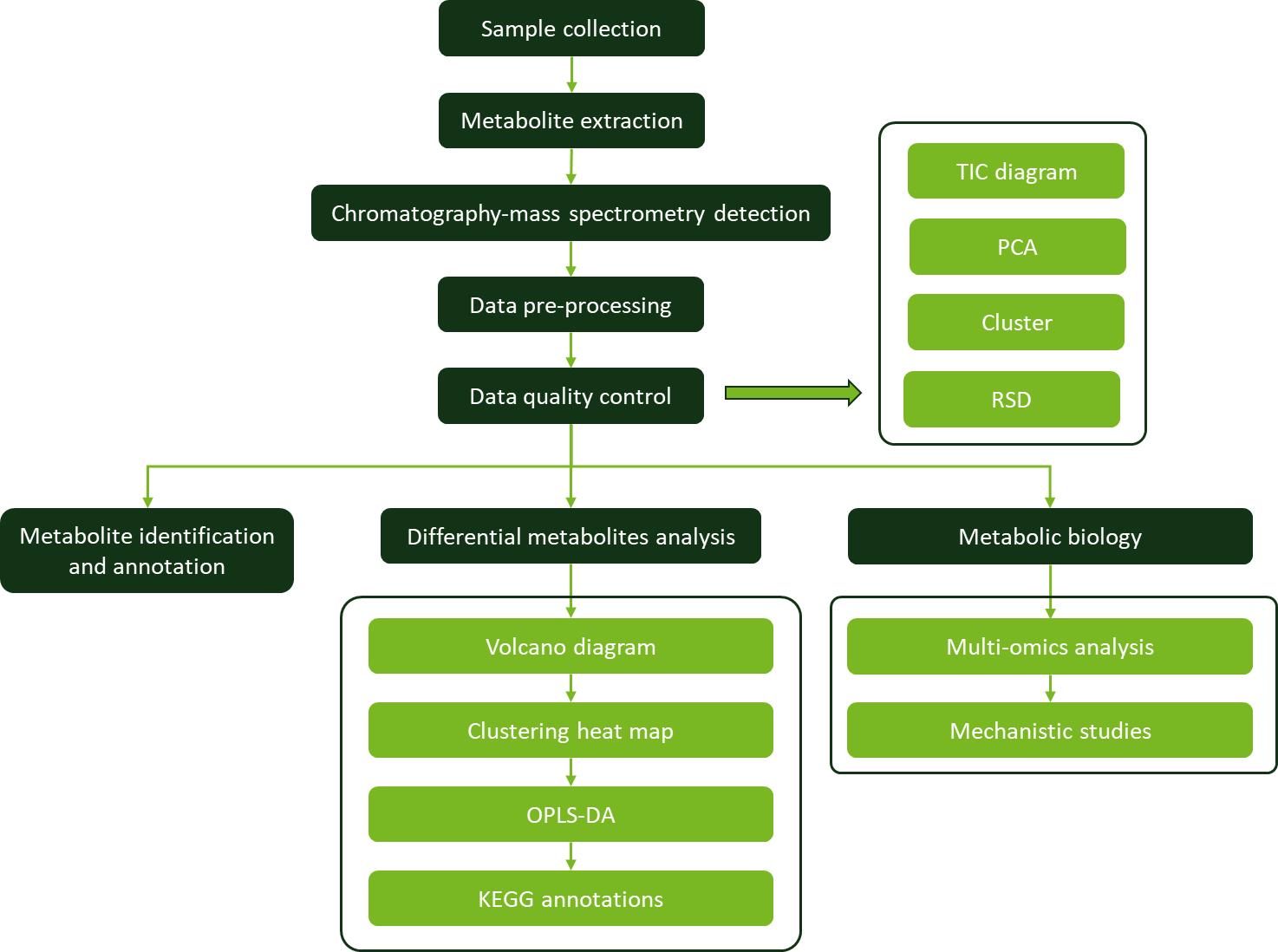 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Myricetin and Its Derivatives Analyzed (including but not limited to)
| Myricetin |
Myricetin 3-O-glucoside |
Myricetin 3-O-galactoside |
Myricetin 3-O-rhamnoside |
| Myricetin 3-O-arabinoside |
Myricetin 3-rutinoside |
Myricetin Hexoside |
Myricetin Pentoside |
| Myricitrin |
|
|
|
Sample Requirements for Myricetin Assay
| Sample Type |
Sample Quantity Required |
| Blood |
1–2 ml |
| Urine |
10–100 ml |
| Plant tissue |
100–1000 mg |
| Food Products |
100–1000 mg |
Deliverables from Myricetin Analysis
Our clients receive:
- A detailed final test report including the method of analysis, analytic results, and an insightful interpretation of results.
- The raw data files of Myricetin's presence and concentration in the sample sent for analysis.
- Personalized consultation for result interpretation and further research planning.
Creative Proteomics is dedicated to offering a comprehensive range of myricetin analysis services using cutting-edge technologies and a team of seasoned analysts. Harness our expertise to fuel your myricetin research endeavors to new heights.
Learn more about other flavonol analysis.
Case. Investigating the Stability of Myricetin in DMEM Using In Situ UPLC–MS–MS Analysis
Background
Flavonols, particularly myricetin, are abundant dietary polyphenols known for their health benefits. However, myricetin's stability during processing and storage is a critical but often overlooked factor. This study aims to address this gap by employing in situ UPLC–MS–MS analysis to investigate the stability of myricetin in Dulbecco's modified Eagle's medium (DMEM) under different temperatures.
Sample
The primary sample investigated in this study is myricetin, a flavonol with a pyrogallol structure. The myricetin standard solution (5 × 10−3 mol/L) was prepared in dimethyl sulfoxide (DMSO). The stability of myricetin was analyzed in DMEM under two temperatures: 37°C and 4°C.
Technical Platform and Procedure
Chemical Preparation:
- Myricetin was purchased and prepared as a standard stock solution (5 × 10−3 mol/L) in DMSO.
- DMEM was used as the medium for stability analysis.
In Situ UPLC–MS–MS Analysis:
- An in situ analysis was conducted using a Thermo Scientific LTQ Orbitrap XL hybrid FT Mass Spectrometer.
- DMEM was prewarmed or precooled, and myricetin standard solution was mixed with DMEM in an autosampler vial.
- A 50.0-µl aliquot was automatically injected into the mass spectrometer every 50 min for multiple cycles.
Reaction Kinetics and Arrhenius Analysis:
- Reaction rates (k1, k2, and k3) were estimated, and the Arrhenius equation was applied to model the temperature dependence of these rates.
- Multivariable fitting using the Runge–Kutta method was employed to analyze the reaction kinetics.
Characterization of New Products:
- MS and MS/MS information were used to deduce the chemical structures of myricetin derivatives (A, B, and C).
- Fragmentation pathways were analyzed to infer the chemical structures of the new products.
Results
Stability in DMEM at 37°C:
- Myricetin was highly unstable at 37°C, with rapid conversion to new products (A and B) within 1 min.
- New product A disappeared after the second injection (50 min), while new product B showed isomer formation and subsequent decline.
Stability in DMEM at 4°C:
- Myricetin demonstrated increased stability at 4°C, with a minor conversion to new product A observed within 1 min.
- Myricetin was no longer detectable after the 14th injection (650 min), indicating enhanced stability at lower temperatures.
Reaction Kinetics:
- Reaction rates (k1, k2, and k3) were determined, revealing that k2 dominated the cascade of consecutive reactions.
- The Arrhenius analysis showed temperature-dependent reaction rates with significant intervals of confidence.
Characterization of New Products:
- New products A and B were identified as derivatives containing the myricetin core structure, with B undergoing oxidation of the ortho-phenolic hydroxyl group.
- New product C consisted of oxidized myricetin as the core
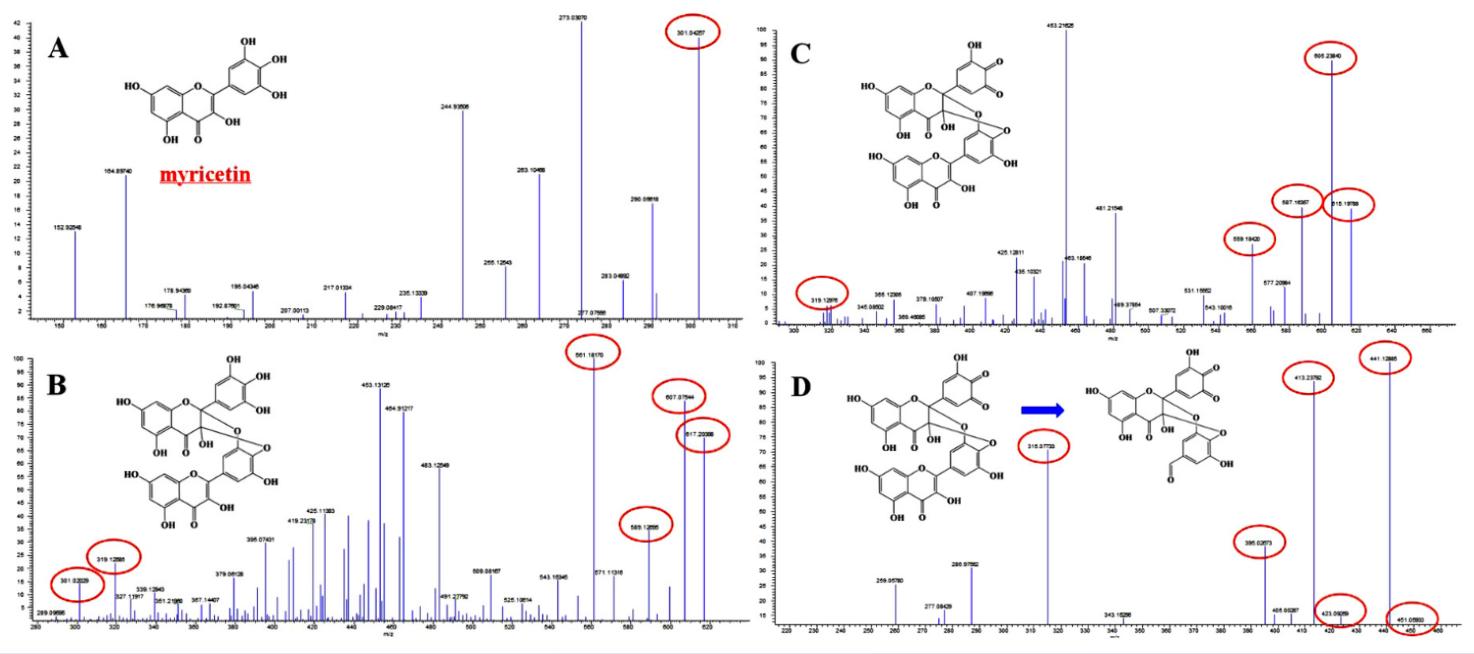 MS–MS chromatogram of new products of myricetin in DMEM
MS–MS chromatogram of new products of myricetin in DMEM
Reference
- Cao, Hui, et al. "Investigation of new products and reaction kinetics for myricetin in DMEM via an in situ UPLC–MS–MS analysis." Food Frontiers 1.3 (2020): 243-252.


 Myricetin
Myricetin Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service MS–MS chromatogram of new products of myricetin in DMEM
MS–MS chromatogram of new products of myricetin in DMEM