What are Polyterpenes?
Polyterpenes are a class of naturally occurring polymers composed of isoprene (C₅H₈) units. These compounds are widely distributed in plants, fungi, and some insects, playing critical roles in biological functions such as membrane stability, signaling, and defense mechanisms.
Polyterpenes include well-known compounds like natural rubber (cis-1,4-polyisoprene), gutta-percha (trans-1,4-polyisoprene), and other structurally diverse terpenoid polymers.
Creative Proteomics offers polyterpene analysis services to support research in biochemistry, plant biology, material sciences, and industrial applications.
Cembrene Analysis Service by Creative Proteomics
Creative Proteomics provides a wide range of specialized services for polyterpenes analysis to ensure high-precision data back your research and development processes. Our service offerings include:
Structural Characterization
- High-resolution MS and MS/MS analysis for polyterpenes structural elucidation
- Fragmentation pattern analysis to determine molecular structure and confirm identity
Polyterpenes ldentification and Quantification
- Targeted GC-MS or LC-MS/MS analysis for accurate quantification of polyterpenes
- Absolute quantification of polyterpenes in biological samples
- Identification of novel polyterpene derivatives
Metabolite Identification & Pathway Mapping
- Detection of polyterpene degradation products
- Mapping of terpenoid biosynthesis pathways
Isotope Tracing Studies
- Combined with mass spectrometry to track the binding of isotopically labelled compounds in metabolic pathways to determine metabolic rates and pathway activity.
Custom Assay Development
- Development and validation of customized polyterpenes quantification assays
- Optimization of extraction and sample preparation protocols for diverse sample matrices
Data Interpretation and Report Generation
- Detailed, scientifically rigorous reports, including all relevant data, trends and analyses
- Assist in decision-making and further research
List of Polyterpenes -Related Metabolites (including but not limited to)
| Polyterpene |
Related Metabolites |
Metabolic Pathways |
| Squalene |
Squalane, Triterpenoids |
Terpenoid biosynthesis, cholesterol biosynthesis |
| Ginsenosides |
Protopanaxadiol, Panaxadiol |
Ginseng saponin biosynthesis |
| Carotenoids |
Retinoids, Lutein |
Isoprenoid biosynthesis, vitamin A synthesis |
| Humulene |
Caryophyllene oxide |
Sesquiterpene biosynthesis |
| Farnesene |
Farnesol, Farnesene oxide |
Terpenoid pathway, pheromone biosynthesis |
| Terpenoid Resins |
Resin acids, Abietic acid |
Resin biosynthesis in conifers |
| Geraniol |
Citronellol, Linalool |
Terpenoid biosynthesis, essential oil production |
Techniques and Instrumentation for Polyterpenes Analysis
SCIEX Triple Quad™ 6500+ (Figure from Sciex)
Mass-Based 1290 Infinity II Preparative LC/MSD System (Figure from Agilent)
Agilent 6495C Triple quadrupole (Figure from Agilent)
Agilent 7890B-5977A (Figure from Agilent)
Workflow for Polyterpenes Analysis Service

Why Choose Us?
- High Sensitivity & Precision: Detection limits down to picomolar levels for trace polyterpenes, high-resolution mass spectrometry ensures isomer differentiation.
- Comprehensive Coverage: Simultaneous detection of polymers, oligomers, and metabolites in a single run.
- Comprehensive Metabolic Profiling: Our service provides not just a measure of polyterpenes but also detailed insights into the entire metabolic pathway leading to its derivatives and final products.
- Speed and Efficiency: Our advanced systems allow for fast processing times, enabling rapid turnaround without compromising quality.
- Scientific Expertise: With over 20 years of experience in biochemical and molecular analysis, our team of experts ensures that your samples are handled with the highest level of scientific integrity.
- Perfect after-sales service: Comprehensive interpretation of the final report, free technical advice, and data review service.
- Comprehensive analyses: 12 analyses, 22 graphical displays.
Applications of Polyterpenes Analysis
|

|
Plant Biology and Biotechnology
Studies on metabolic engineering of rubber biosynthesis and promotion of terpenoid production in Brazilian rubber trees
|

|
Materials Science
Quality control of natural rubber products and characterisation of bio-based polymers
|
|

|
Industrial Biotechnology
Optimization of microbial terpenoid production and analysis of biodegradable materials
|

|
Drug Development
Explore the characterization and biosynthesis of polyterpenes for drug development
|
Sample Requirements for Polyterpenes Analysis Assay
| Sample Types |
Minimum Quantity |
Storage & Handling |
| Plant Tissues |
100 mg (fresh) |
Flash-freeze in liquid N₂ |
| Latex/Rubber |
50 mg |
Store at -80°C, avoid UV exposure |
| Extracts (Lipid Fraction) |
200 µL |
Store in amber vials at -20°C |
| Synthetic Polymers |
10 mg |
Dry powder, desiccated |
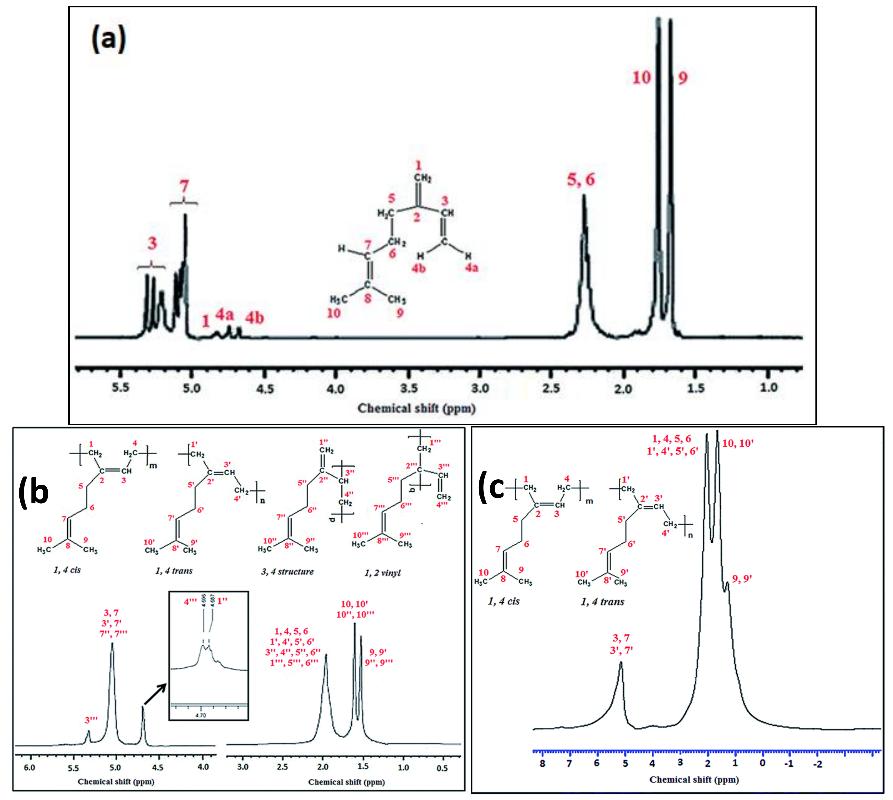 (a) 1H NMR spectrum of β-myrcene, (b) and (c) 1H NMR spectra of polymyrcene (persulfate and redox initiated). (Figure from Sarkar, P et al., 2014)
(a) 1H NMR spectrum of β-myrcene, (b) and (c) 1H NMR spectra of polymyrcene (persulfate and redox initiated). (Figure from Sarkar, P et al., 2014)
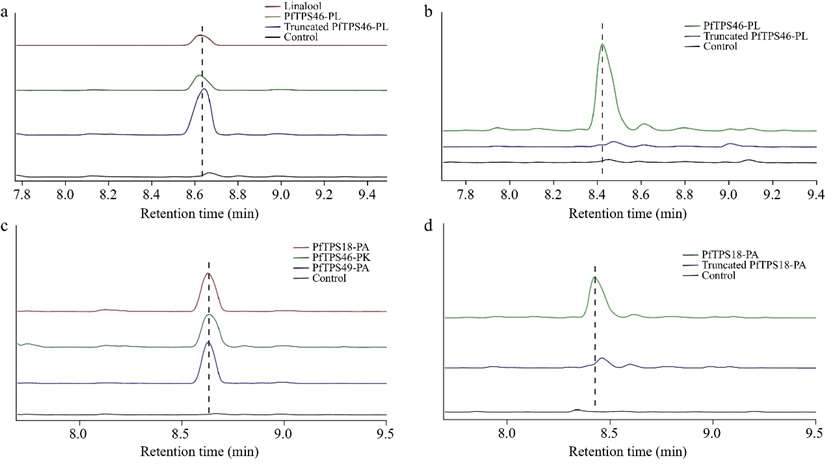 Functional characterization of four PfTPS enzymes. (a) Heterologous functional characterization and (b) in vitro enzymatic reaction of PfTPS46-PL. (c) Heterologous functional characterization and (d) in vitro enzymatic reaction of PfTPS46-PK, PfTPS18-PA, and PfTPS49-PA. (Figure from Shi Mei Yang et al., 2024)
Functional characterization of four PfTPS enzymes. (a) Heterologous functional characterization and (b) in vitro enzymatic reaction of PfTPS46-PL. (c) Heterologous functional characterization and (d) in vitro enzymatic reaction of PfTPS46-PK, PfTPS18-PA, and PfTPS49-PA. (Figure from Shi Mei Yang et al., 2024)
Case. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata
Background:
- Aralia elata for Araliaceae Aralia species perennial shrubs, is mainly produced in China's northeastern forested areas of the important food and medicine homologous plants, also known as the "king of the wild vegetables", with anti-cancer, anti-inflammatory and antioxidant effects, triterpene saponins is the main active ingredient.
- This study sheds light on the diversity of saponins biosynthetic pathway in Araliaceae and will facilitate heterologous bioproduction of aralosides.
Samples:
- Fresh leaf tissue of Aralia elata
- Roots, stems and leaves of two-year old P. ginseng, P. notoginseng plants and five-year old A. elata plants
- Three biological replicates of each plant and six plants per biological replicate
Technical methods procedure:
- High-quality genome mapping of Aralia elata using PacBio triple sequencing and Hi-C technology for comparative genomic analysis.
- Total RNA was extracted from the samples using the RNA Extraction Kit and then sequenced on an Illumina HiSeq 4000 sequencer and analyzed for transcriptomics.
- Samples of roots, stems and leaves of P. ginseng plants, P. notoginseng plants and A. elata were uniformly mixed.
- Metabolites were extracted from each sample using 80% ethanol.
- The metabolites were identified and quantified by HPLC-UV-MS/MS.
- Ab initio synthesis of 13 aralia saponins using Saccharomyces cerevisiae as chassis cells.
Results:
- Exon deletion of DDS (dammarane diol synthase), a key enzyme gene responsible for catalysing dammarane-type saponins in the genome of Aralia elata, resulted in the inability to synthesise dammarane-type saponins in A. elata.
- Structural analysis of the genome showed that a large number of tandem duplications of triterpenoid saponin modifying enzyme genes occurred in the genome of Aralia after its divergence from Panax.
- The transcriptome, metabolome and physiological and biochemical assays identified several key enzymes catalysing the synthesis of A. elata triterpenoid saponins, and systematically analysed the biosynthetic pathway of araloside of oleanoid type.
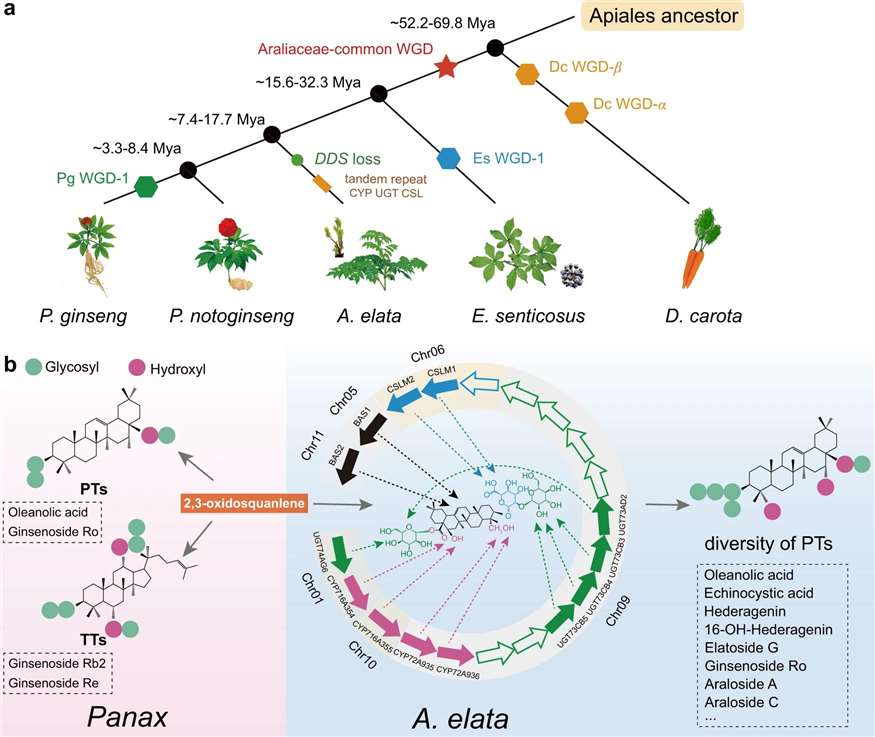 Phylogeny of Araliaceae medicinal plants and the mechanism of triterpenoid saponin diversity formation. (Figure from Yu Wang et al., 2022)
Phylogeny of Araliaceae medicinal plants and the mechanism of triterpenoid saponin diversity formation. (Figure from Yu Wang et al., 2022)
Reference
- Wang, Y., Zhang, H., Ri, H.C. et al. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. Nat Commun 13, 2224 (2022). https://doi.org/10.1038/s41467-022-29908-y










 (a) 1H NMR spectrum of β-myrcene, (b) and (c) 1H NMR spectra of polymyrcene (persulfate and redox initiated). (Figure from Sarkar, P et al., 2014)
(a) 1H NMR spectrum of β-myrcene, (b) and (c) 1H NMR spectra of polymyrcene (persulfate and redox initiated). (Figure from Sarkar, P et al., 2014) Functional characterization of four PfTPS enzymes. (a) Heterologous functional characterization and (b) in vitro enzymatic reaction of PfTPS46-PL. (c) Heterologous functional characterization and (d) in vitro enzymatic reaction of PfTPS46-PK, PfTPS18-PA, and PfTPS49-PA. (Figure from Shi Mei Yang et al., 2024)
Functional characterization of four PfTPS enzymes. (a) Heterologous functional characterization and (b) in vitro enzymatic reaction of PfTPS46-PL. (c) Heterologous functional characterization and (d) in vitro enzymatic reaction of PfTPS46-PK, PfTPS18-PA, and PfTPS49-PA. (Figure from Shi Mei Yang et al., 2024) Phylogeny of Araliaceae medicinal plants and the mechanism of triterpenoid saponin diversity formation. (Figure from Yu Wang et al., 2022)
Phylogeny of Araliaceae medicinal plants and the mechanism of triterpenoid saponin diversity formation. (Figure from Yu Wang et al., 2022)