What is Proanthocyanidin?
Proanthocyanidins, also known as condensed tannins, are a specific type of flavonoid. They are oligomeric flavonoids, consisting of flavan-3-ol units linked together to form more complex structures. Proanthocyanidins are characterized by their capacity to form red complexes with ferric chloride, their reactivity with acid vanillin reagents, and their astringent taste.
Proanthocyanidins have been found in various plant species, with particularly high concentrations in the skins and seeds of grapes, the bark of pine trees, and the leaves of bilberry and cranberry plants. They have potent antioxidant activities and are thought to exhibit health-enhancing properties such as anti-inflammatory, antiviral, and anticancer activities. Several studies have linked the intake of Proanthocyanidin-rich foods to reduced risks of chronic illnesses, such as cardiovascular disease and various forms of cancer.
Understanding the proanthocyanidin content in food and dietary supplements is important for diet planning. Accurately quantifying and identifying the specific types of proanthocyanidin contained can enable manufacturers to enhance the health benefits of their products, in addition to maintaining a consistently high-quality output for consumers.
At Creative Proteomics, we recognize the importance of reliable and comprehensive proanthocyanidin analysis, which propels us to offer specialized services in this direction. Utilizing advanced mass spectrometry techniques, our team of experts provides insights into the wide array of proanthocyanidin in different sample types, with a firm commitment to precision, accuracy, and reproducibility.
Specific Project Offered by Creative Proteomics in Proanthocyanidin Analysis
Targeted Proanthocyanidin Analysis: Crafted for specific compounds of interest, these projects deliver detailed insights into the presence and concentration of particular proanthocyanidins. Ideal for investigating plant extracts or analyzing the composition of specific food products.
Multi-Sample Comparative Analysis: Designed for industries like agriculture and food production, this service allows clients to compare proanthocyanidin profiles across different samples. Identifying trends and differences facilitates optimization and product development.
Longitudinal Proanthocyanidin Studies: For research and development, these projects involve repeated analysis over time, offering dynamic insights into changes in proanthocyanidin composition. Essential for tracking stability, understanding processing impacts, and ensuring product consistency.
Customized Analytical Method Development: Creative Proteomics excels in developing tailored analytical methods, collaborating closely with clients to address specific challenges. This ensures optimal sensitivity, selectivity, and accuracy in proanthocyanidin analysis, even for complex matrices.
Comprehensive Proanthocyanidin Profiling: Going beyond basic analysis, this service provides a detailed map of the entire proanthocyanidin spectrum in a sample. Beneficial for industries requiring a deep understanding of proanthocyanidin diversity and complexity.
Proanthocyanidin Analysis Techniques
We rely on cutting-edge Mass Spectrometry based techniques to perform proanthocyanidin analysis. The instruments we use include:
- MALDI-TOF-MS (Matrix-Assisted Laser Desorption/Ionization - Time-of-Flight Mass Spectrometry)
- LC-MS/MS (Liquid Chromatography–Tandem Mass Spectrometry)
These techniques offer high sensitivity, accurate quantification, and comprehensive coverage.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Proanthocyanidin Analyzed (including but not limited to)
| Procyanidin A2 |
Procyanidin B1 |
Procyanidin B2 |
Procyanidin B3 |
| Procyanidin B4 |
Procyanidin C1 |
B-Pinene |
Catechin |
| Epicatechin |
Epicatechin gallate |
Taxifolin |
|
Sample Requirements for Proanthocyanidin Assay
| Sample Type |
Recommended Quantity |
| Plant tissues |
100-200 mg |
| Fruits |
100 mg |
| Nuts |
100 mg |
| Beverages |
2 mL |
| Dietary supplements |
100 mg |
| Animal Tissues |
100-200 mg |
| Biological Fluids |
200-500 µL |
Case. Comprehensive Analysis of Cranberry Proanthocyanins: Structural Diversity and Novel Derivatives
Background
Cranberry proanthocyanins (PACs) play a crucial role in the health benefits associated with cranberry consumption. However, a detailed understanding of their structural diversity and the presence of novel derivatives remains limited. This study aims to unravel the complex composition of cranberry PACs through advanced analytical techniques.
Sample
The study utilized cranberry samples, specifically the OSA7 extract, for in-depth analysis. Different fractions (F1 to F6) obtained through normal-phase HPLC fractionation were scrutinized to uncover the intricate details of the PAC composition.
Technical Platform and Procedure
1. Positive Ion Mode Analysis: Mass spectrometry in positive ion mode revealed that fractions F1 to F3 lacked anthocyanin ethyl-bridged PACs, which were identified in F4 to F6. Different flavanol moieties (monomer to tetramer) were observed, and retention time variations suggested the presence of PAC isomers with diverse A-type and ethyl bond positions.
2. Fragmentation Analysis: Fragmentation studies provided structural insights, such as the identification of peonidin-hexoside-ethyl-(epi)catechin-A2 and a mixture of related compounds. Thiolysis degradation confirmed the resistance of certain structures and indicated challenges in interflavan bond cleavage for DP3 and DP4 PAC moieties with an A-type linkage.
3. Pyranoanthocyanin Derivatives: Fractions F5 and F6 contained pyranoanthocyanin derivatives, possibly formed through reactions with 8-vinylflavanol derivatives, exhibiting a hypsochromic shift in the visible spectrum.
4. Negative Ion Mode Analysis: Flavonol-ethyl-flavanol derivatives were detected in fraction F3, including compounds like (epi)catechin-ethyl-quercetin-arabinoside.
Results
Positive Ion Mode Findings: Detailed identification of flavanol moieties (monomer to tetramer) linked to anthocyanins in F4 to F6 fractions. Isomeric variations and ethyl bridge positions were observed.
Fragmentation Insights: Structural confirmation through fragmentation studies, revealing specific compounds like peonidin-hexoside-ethyl-(epi)catechin-A2 and related mixtures.
Pyranoanthocyanin Discoveries: Identification of pyranoanthocyanin derivatives in F5 and F6, characterized by a hypsochromic shift in the visible spectrum.
Negative Ion Mode Highlights: Detection of flavonol-ethyl-flavanol derivatives in F3, including compounds like (epi)catechin-ethyl-quercetin-arabinoside.
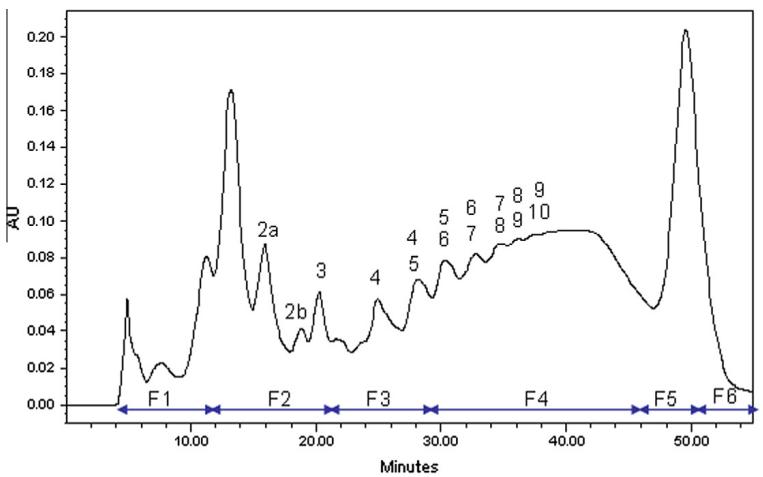 UV–Vis diol-phase HPLC chromatogram of the OSA7 extract at 280 nm
UV–Vis diol-phase HPLC chromatogram of the OSA7 extract at 280 nm
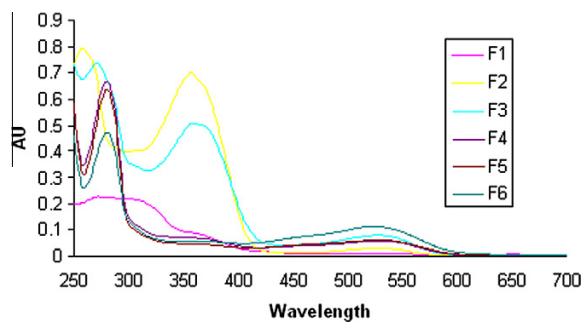 UV–Vis spectra recorded for the OSA7 fractions
UV–Vis spectra recorded for the OSA7 fractions
Reference
- Tarascou, Isabelle, et al. "Characterisation of genuine and derived cranberry proanthocyanidins by LC–ESI-MS." Food Chemistry 128.3 (2011): 802-810.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service UV–Vis diol-phase HPLC chromatogram of the OSA7 extract at 280 nm
UV–Vis diol-phase HPLC chromatogram of the OSA7 extract at 280 nm UV–Vis spectra recorded for the OSA7 fractions
UV–Vis spectra recorded for the OSA7 fractions