What is Homoeriodictyol?
Homoeriodictyol is a flavonoid compound commonly found in various plant species, including Aloe and Scutellaria. It exhibits a range of biological activities, including antioxidant, anti-inflammatory, antimicrobial, and anticancer properties.
The significance of analyzing Homoeriodictyol lies in its potential medicinal value and widespread occurrence in medicinal plants. By quantifying its content, researchers and manufacturers can assess the potency and consistency of herbal products or pharmaceutical formulations containing Homoeriodictyol. Moreover, understanding the levels of Homoeriodictyol in plants can facilitate scientific investigations into its pharmacological mechanisms and its potential applications in health and disease management.
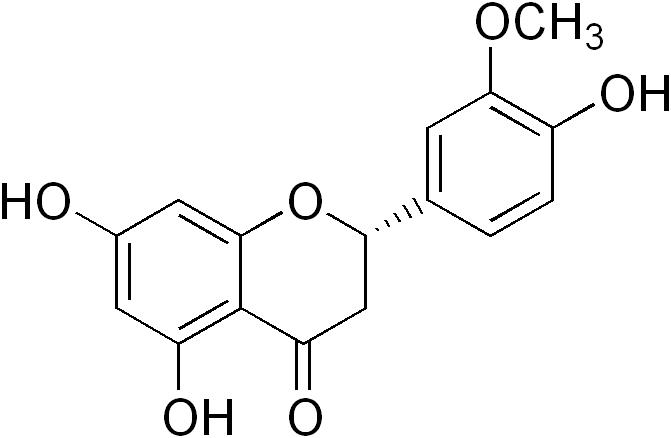 Homoeriodictyol
Homoeriodictyol
Creative Proteomics offers specialized homoeriodictyol analysis services tailored to the needs of researchers and product developers. Our state-of-the-art laboratories ensure high-quality, accurate results.
Homoeriodictyol Analysis Offered by Creative Proteomics
Homoeriodictyol Quantitative and Qualitative Analysis
- High-Performance Liquid Chromatography (HPLC): Provides quantification and purification of homoeriodictyol from various samples, ensuring precise measurements.
- Liquid Chromatography-Mass Spectrometry (LC-MS/MS): Offers sensitive detection and quantification of homoeriodictyol, even at low concentrations, and provides structural insights.
Homoeriodictyol Metabolic Profiling
- Targeted Metabolomics: Investigates the metabolic profiles related to homoeriodictyol to understand its bioactivity and pharmacokinetics, aiding therapeutic and nutraceutical development.
Homoeriodictyol Stability and Degradation Studies
- Stability Testing: Evaluates homoeriodictyol stability under different environmental conditions such as light, temperature, and pH.
- Degradation Analysis: Identifies degradation products and their formation conditions to improve product shelf-life and efficacy.
Homoeriodictyol Bioavailability and Pharmacokinetics
- ADME Studies: Assesses how homoeriodictyol is absorbed, metabolized, and excreted using sophisticated in vitro and in vivo models.
Homoeriodictyol Regulatory Compliance Support
- Documentation and Validation: Provides comprehensive documentation and validation services to meet international regulatory standards, ensuring product compliance.
Custom Analytical Services
- Custom Method Development and Validation: Offers tailored method development and validation to meet specific project requirements.
Technological Platforms for Homoeriodictyol Analysis
High-Performance Liquid Chromatography (HPLC): HPLC is utilized for the precise quantification of homoeriodictyol, with methods optimized for peak resolution and separation efficiency, critical for accurate analysis in complex biological and botanical extracts.
Our labs are equipped with the latest HPLC systems, including the Agilent 1260 and 1290 Infinity series, which are known for their robust performance and reliability. These systems are coupled with UV, DAD (Diode-Array Detection), and fluorescence detectors, allowing for high sensitivity and selectivity in the detection of homoeriodictyol across various sample matrices.
Liquid Chromatography-Mass Spectrometry (LC-MS/MS): LC-MS/MS is used for identification and quantification, providing detailed mass spectrometry data that helps confirm the presence and structure of homoeriodictyol. We utilize the Thermo Fisher Scientific Orbitrap and Triple Quadrupole systems to provide high-resolution accurate mass measurements and superior quantitation capabilities. These instruments are essential for sensitive and specific detection of homoeriodictyol, even at low concentrations.
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy is employed for structural elucidation and confirmation of homoeriodictyol. This technique allows for the determination of molecular connectivity, stereochemistry, and conformational analysis. By acquiring 1D and 2D NMR spectra, including ^1H, ^13C, COSY, HSQC, and HMBC experiments, we can confidently identify homoeriodictyol and elucidate its chemical structure with high precision.
Data Analysis and Interpretation: Creative Proteomics employs advanced data analysis software and techniques to process and interpret the results obtained from various analytical platforms. Our team of experienced scientists utilizes statistical analysis, multivariate data analysis, and database searching to extract meaningful information from complex datasets, enabling comprehensive characterization and quantification of homoeriodictyol.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Homoeriodictyol Assay
| Sample Type |
Sample Volume |
Sample Preparation |
| Biological Samples (e.g., plasma, serum, urine) |
100-500 µL |
- Centrifuge at 10,000 rpm for 10 minutes to remove debris
- Transfer supernatant for analysis |
| Botanical Extracts |
10-100 mg |
- Dilute with appropriate solvent (e.g., methanol, ethanol)
- Filter through a 0.2 µm membrane filter prior to analysis |
| Dietary Supplements |
50-200 mg |
- Dissolve in suitable solvent (e.g., methanol, acetonitrile)
- Centrifuge if necessary to remove insoluble particles |
| Pharmaceutical Formulations |
As per dosage form |
- Extract homoeriodictyol using appropriate extraction method
- Filter prior to analysis |
| Food Samples |
1-10 g |
- Homogenize sample thoroughly
- Extract homoeriodictyol using suitable solvent
- Centrifuge and filter before analysis |
Applications of Homoeriodictyol Analysis
Pharmaceutical Industry
- Drug Development: Homoeriodictyol analysis plays a crucial role in drug discovery and development, especially in the evaluation of its pharmacological activities and potential therapeutic effects. Understanding its bioavailability, metabolism, and pharmacokinetics aids in the development of novel pharmaceutical formulations targeting various diseases.
- Quality Control: Analysis of homoeriodictyol ensures the quality and consistency of pharmaceutical products containing this compound. It helps in verifying the presence and concentration of homoeriodictyol in drug formulations, ensuring compliance with regulatory standards.
Academic Research
- Phytochemical Studies: Homoeriodictyol analysis is utilized in phytochemical studies aimed at elucidating the composition and bioactivity of natural plant extracts. It contributes to the identification and characterization of homoeriodictyol in various plant sources, facilitating further research into its biological functions and potential therapeutic applications.
- Metabolomics Research: Homoeriodictyol analysis is integrated into metabolomics studies to explore its metabolic pathways and interactions within biological systems. This provides valuable insights into the physiological effects of homoeriodictyol and its potential role in health and disease.
Nutraceuticals and Dietary Supplements
- Health Benefits Assessment: Homoeriodictyol analysis is utilized to evaluate the presence and concentration of this compound in nutraceuticals and dietary supplements. It enables manufacturers to quantify the bioactive component and assess its potential health benefits, such as antioxidant and anti-inflammatory properties.
- Formulation Development: Understanding the concentration of homoeriodictyol in dietary supplements allows for the optimization of formulations to enhance its bioavailability and efficacy, ensuring maximum health benefits for consumers.
Food and Beverage Industry
- Functional Food Development: Homoeriodictyol analysis supports the development of functional foods enriched with bioactive compounds. By quantifying homoeriodictyol content in food products, manufacturers can label and market their products based on their health-promoting properties, attracting health-conscious consumers.
- Quality Assurance: Analysis of homoeriodictyol ensures the quality and authenticity of food products claiming to contain this compound. It helps in detecting adulteration and ensuring compliance with food safety regulations.
Case. Development and Validation of HPTLC-Densitometric Method for Simultaneous Quantification of Persicogenin and Homoeriodictyol in Rhus Species
Background
The genus Rhus, commonly known as Sumac, comprises over 250 species with widespread distribution. Various species of Rhus have been traditionally used for medicinal purposes, including treatment of syphilis, gonorrhea, and wound healing. Persicogenin and homoeriodictyol are flavonoid compounds isolated from R. retinorrhoea, exhibiting pharmacological activities such as anticancer and antioxidant properties. However, a validated analytical method for simultaneous quantification of these compounds in Rhus species is lacking.
Sample
Aerial parts of R. retinorrhoea and R. tripartita were collected from the northern region of Saudi Arabia and authenticated by a field taxonomist. Methanol extracts of the samples were used for analysis.
Technical Methods
Sample Preparation: Aerial parts were air-dried, pulverized, and subjected to continuous hot extraction with methanol. The methanol extract was concentrated and vacuum-dried.
Standard Preparation: Homoeriodictyol and persicogenin standards were isolated from R. retinorrhoea and dissolved in methanol. Calibration solutions were prepared in the range of 100–800 ng per band.
HPTLC Analysis: Chromatographic analysis was performed using 20 × 10 cm HPTLC plates. Samples were applied as bands, developed in toluene–ethyl acetate–methanol mobile phase, and quantified at 293 nm using densitometry.
Method Validation: Validation parameters included linearity, precision, accuracy, robustness, LOD, LOQ, and recovery. Precision was evaluated at three QC levels, and accuracy was determined by the standard addition method. Robustness was assessed by introducing small changes in the chromatographic conditions.
Results
The developed HPTLC-densitometric method demonstrated good linearity, precision, accuracy, and robustness. Homoeriodictyol and persicogenin were detected in the methanol extract of R. retinorrhoea but not in R. tripartita. The method provided a precise and selective means for the simultaneous quantification of these compounds in Rhus species, paving the way for further research and quality control applications.
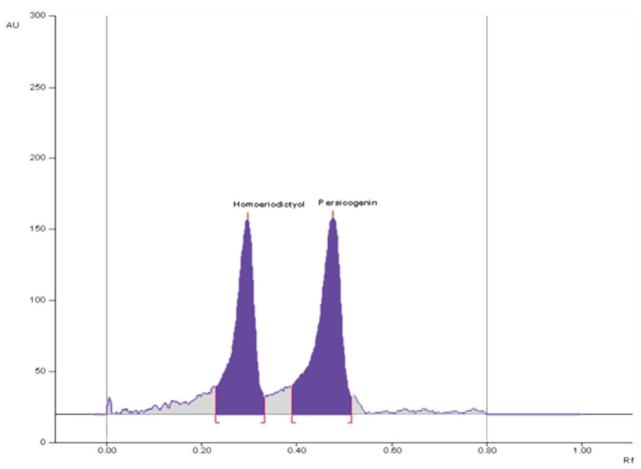 Chromatogram of standards homoeriodictyol (700 ng spot–1; RF =0.30) and persicogenin (700 ng spot–1; RF = 0.48) with mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
Chromatogram of standards homoeriodictyol (700 ng spot–1; RF =0.30) and persicogenin (700 ng spot–1; RF = 0.48) with mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
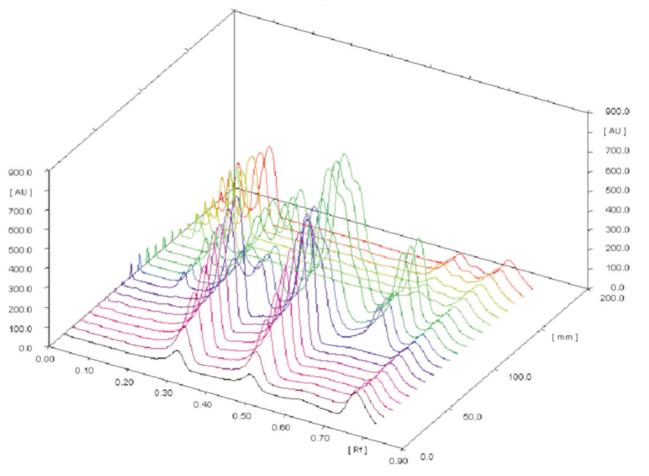 3D display of all tracks at 293 nm; mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
3D display of all tracks at 293 nm; mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
Reference
- Siddiqui, Nasir, et al. "Simultaneous quantification of two bioactive flavonoids, homoeriodictyol and persicogenin, in the methanol extract of the aerial parts of two different species of genus Rhus by a validated high-performance thin-layer chromatographic-densitometric method." JPC-Journal of Planar Chromatography-Modern TLC 28.1 (2015): 42-47.


 Homoeriodictyol
Homoeriodictyol Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Chromatogram of standards homoeriodictyol (700 ng spot–1; RF =0.30) and persicogenin (700 ng spot–1; RF = 0.48) with mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
Chromatogram of standards homoeriodictyol (700 ng spot–1; RF =0.30) and persicogenin (700 ng spot–1; RF = 0.48) with mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v). 3D display of all tracks at 293 nm; mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).
3D display of all tracks at 293 nm; mobile phase: toluene–ethyl acetate–methanol (8:2:0.5, v/v).