DIA MS Host Cell Protein Profiling Solutions
Host cell proteins (HCPs) constitute a major class of impurities that must be monitored and efficiently removed during the biopharmaceutical protein purification process. Remaining HCPs in the final drug product can reduce the drug efficacy or induce adverse patient reactions. Therefore, HCP amounts in the final drug product must be provided to the regulatory authorities. Biopharmaceutical proteins typically undergo a series of purification steps to remove contaminants. Depending on the application of the protein, there are different requirements for purity, but host cell proteins (HCPs) generally remain at trace levels.
The Method of HPCs Monitoring
ELISA and data-dependent (DDA)LC-MS methods are commonly used for HCP analysis of biopharmaceuticals because they can identify and quantify individual species. However, there is increasing evidence that ELISA cannot provide comprehensive HCP quantification. Because it only detects HCPs that induce animal immune responses during the ELISA development process and only includes information about the total HCP amounts, but cannot identify the identity information of the detected HCPs.
As an alternative, data-dependent mass spectrometry (MS) approaches were recently revealed to be most promising to characterize HCP contents as they allow unbiased quantification and individual HCP monitoring. But, conventional data-dependent LC-MS was affected by low concentrations of HCP-derived peptides, which were 5-6 orders of magnitude lower compared to biologic drug-derived peptides. More recently, data-independent acquisition (DIA) based mass spectrometry methods has been reported to confirm the absolute quantification of HCP-derived peptides at low concentrations.
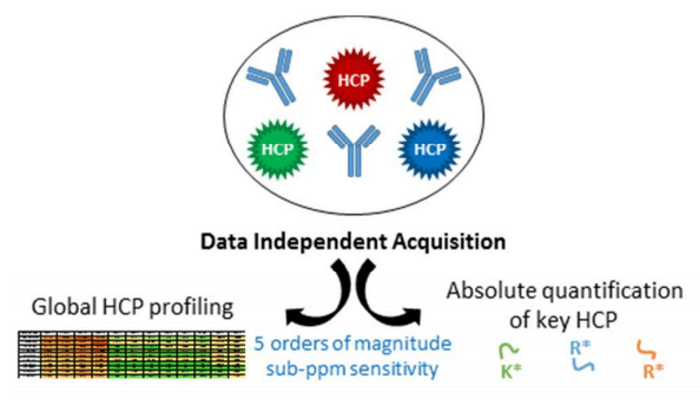
Our DIA-based HCPs Services
Utilizing DIA-MS Platforms, Creative Proteomics offers HCP analysis service packages. Of course, as a leader in the field of proteomics, we can also provide ELISA and conventional mass spectrometry services for HPCs detection according to your needs. All you need to do is send us your samples and tell us your needs and we will take care of all the follow-ups of your project, including protein extraction, proteolysis, peptide separation, mass spectrometry analysis, raw mass spectrometry data analysis, and bioinformatics analysis.
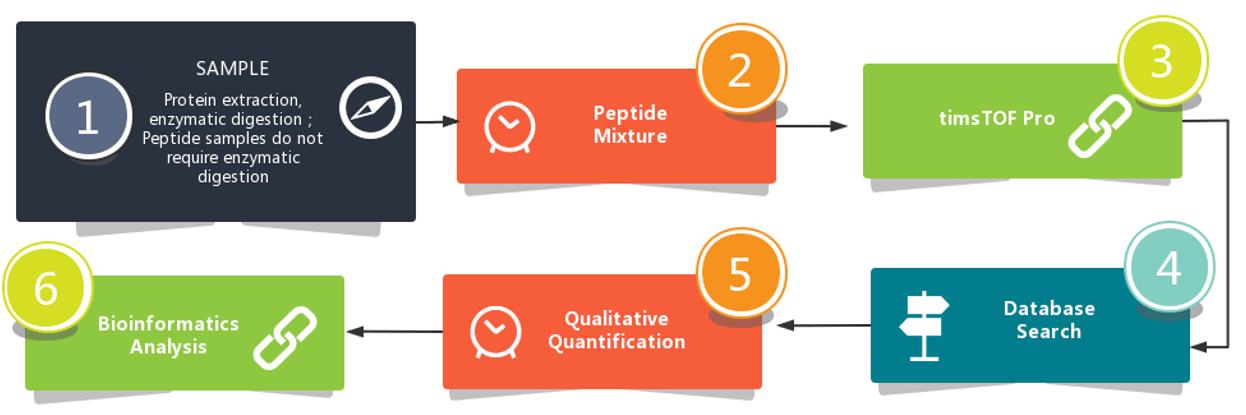
Workflow
The depth and relatively straightforward nature of our workflow should make it a powerful new tool in detecting HCPs of biopharmaceuticals.
Report
- Experimental steps
- Relevant experiment parameters
- Mass spectrometry pictures
- Raw data
- Proteomics analysis results
Since we started to provide DIA technology service, we have accumulated a lot of experience not only in the detection of a large cohort of conventional samples, but also in providing one-stop service of DIA+PRM+machine learning for biomarker studies, tumor-based proteomic typing and large-scale clinical sample proteomics analysis based on next-generation chromatography (Evosep DIA). Free consultation is welcome.
References:
- Husson, Gauthier, et al. Dual Data Independent Acquisition approach combining global HCP profiling and absolute quantification of key impurities during bioprocess development. Analytical Chemistry. 2017.
- Søren Heissel, et al. Evaluation of spectral libraries and sample preparation for DIA-LC-MS analysis of host cell proteins: A case study of a bacterially expressed recombinant biopharmaceutical protein.Protein Expr Purif 147:69-77 2018.


 4D Proteomics with Data-Independent Acquisition (DIA)
4D Proteomics with Data-Independent Acquisition (DIA)