4D-Glycosylation Proteomics Services
Glycosylation is the process of transferring glycan to proteins under the action of glycosyltransferases to form glycosidic bonds with amino acid residues. Glycosylation is an important protein modification and plays a crucial role in the proper folding, functional localization, and intracellular transport of proteins.
Among all glycosylation modifications, N-glycosylation is one of the most widely studied cases. N-glycosylation involves in the essential role of sugar chains in various life activities, including cell recognition, cell differentiation, signal transduction, and immune response by linking with the free asparagine's NH2 group of proteins. Moreover, N-glycosylation is significant in the research of embryonic development, cancer development, and immune defense and defense against pathogenic bacteria invasion.
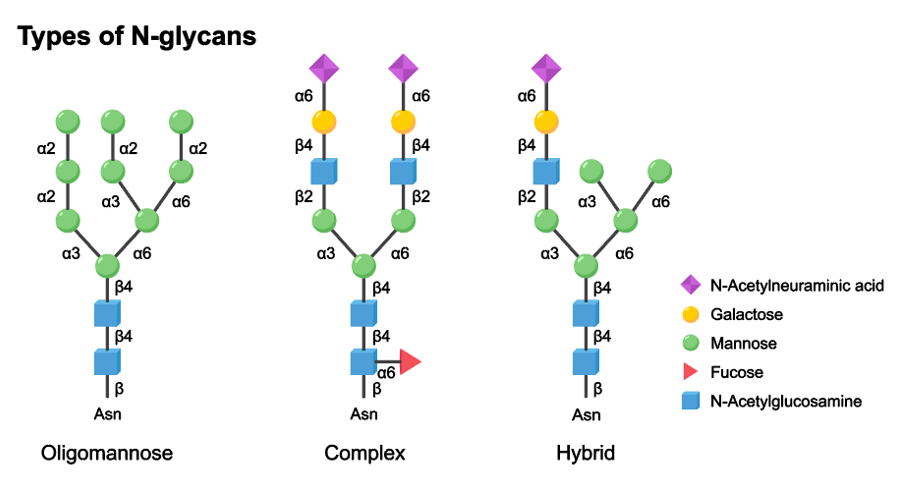
Our 4D-Glycosylation Proteomics Services
Creative Proteomics uses the novel generation of 4D platform to provide qualitative and quantitative analysis for protein glycosylation and modification sites, which significantly improves sensitivity and depth of coverage. The mass spectrometry technique and enrichment processing allow for large throughput detection of glycosylation-modified proteins with low levels and a wide dynamic range. We provide "one-stop" proteomics services, from large-scale PTMs proteomics screening, PRM validation of targeted proteomics/PTMs omics, to high-quality antibody-assisted validation and subsequent molecular mechanism studies.
Workflow
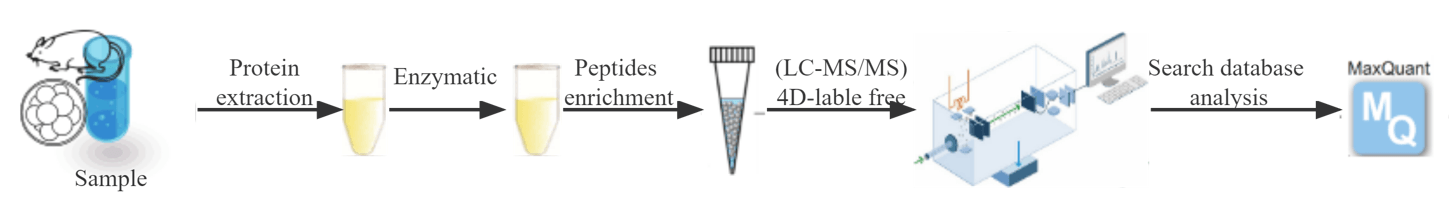
Applications
- Disease mechanism research, disease marker discovery, disease molecular typing
- Therapeutic targets screening, drug toxicology, cellular regulation, immune defense, etc.
- Research on resistance to disease, stress and mechanisms of growth and development
- Breeding protection research, storage and processing conditions optimization, etc.
Advantages
- Enormous research potential: novel O-glycan modification, a hot spot for glycobiology research and high application potential
- Cutting-edge technology platform: new generation 4D platform, higher identification depth and more accurate site analysis
- Experimental data guarantee: gold standard antibody and high standard enrichment operation to ensure the stability of experimental process
- One-stop analysis: all O-GlcNAc sites and glycoforms are analyzed and can be combined with phosphorylation to achieve a one-stop analysis
Data Analysis
| Standard Data Analysis | |
| Analysis of total protein identification quantity | Protein identification and quantitative result statistics, Venn diagram analysis, number of different results statistics, volcano map, cluster heat map |
| Protein function analysis | Subcellular localization, domain analysis, motif analysis, GO function analysis, KEGG pathway analysis, interaction network analysis |
| Quality control analysis | Peptide relative molecular mass distribution, peptide sequence length distribution |
Sample Requirements
Cell: 5×107-108
Protein: >800 µg
Cell collection (try to avoid trypsin); phosphatase inhibitors are required throughout sample processing; protein samples are transported on dry ice.
Instruments
THERMO Q-EXACTIVE HF,BRUKER TIMS TOF PRO
Report
- Experimental steps
- Relevant experimental parameters
- Mass spectrometry spectra
- Raw data
- Proteomics analysis results
References:
- Quantitative chemoproteomics reveals OGlcNAcylation of cystathionine g-lyase (CSE) represses trophoblast syncytialization. 2021.Cell chemical biology.
- A mutant O-GlcNAcase enriches Drosophila developmental regulators. 2017.Nature chemical biology.
- The Protein Modifications of O-GlcNAcylation and Phosphorylation Mediate Vernalization Response for- Flowering in Winter Wheat. 2019. Plant physiology.


 4D Proteomics with Data-Independent Acquisition (DIA)
4D Proteomics with Data-Independent Acquisition (DIA)