What is Organelle Omics?
The cellular world is a complex interplay of various organelles, each with its own set of functions and significance. Organelle omics analysis is a revolutionary approach in cellular biology that involves the comprehensive study of cellular organelles at the genomic, transcriptomic, proteomic, and metabolomic levels. By scrutinizing the genetic, RNA, protein, and metabolic profiles of specific organelles, this multidimensional analysis provides a nuanced understanding of their functions and interactions within the cellular context. The integration of data from various omics layers enables researchers to unravel the intricate molecular dynamics of organelles, contributing to a holistic comprehension of cellular processes, health, and disease. This cutting-edge methodology holds immense promise for advancing our knowledge in cell biology and fostering breakthroughs in diverse scientific disciplines.
Overview of Cellular Organelles:
Exosome: Exosomes, small membrane vesicles, play a crucial role in intercellular communication. Organelle omics sheds light on the molecular cargo carried by exosomes, revealing insights into cell signaling and disease mechanisms.
Membrane: The cell membrane forms the boundary between a cell and its environment. Organelle omics provides a comprehensive understanding of membrane composition, offering clues about cellular interactions and responses to external stimuli.
Mitochondrial: Mitochondria, the powerhouse of the cell, are central to energy production. Organelle omics analysis unveils the mitochondrial proteome, aiding in the exploration of energy metabolism, cellular respiration, and implications in various diseases.
Chloroplast: Exclusive to plant cells, chloroplasts are responsible for photosynthesis. Organelle omics studies unravel the intricacies of chloroplast function, providing insights into plant biology, crop improvement, and environmental responses.
Nuclear: The nucleus houses genetic material and orchestrates cellular activities. Organelle omics techniques delve into nuclear genomics, offering a glimpse into gene expression, regulation, and the intricate dance of molecules within the nucleus.
Peroxisome: Peroxisomes contribute to cellular homeostasis through redox reactions. Organelle omics allows for the identification of peroxisomal enzymes and metabolites, aiding in understanding their role in detoxification and lipid metabolism.
Golgi Apparatus: The Golgi apparatus is involved in processing and packaging proteins. Organelle omics examines the protein modifications occurring in the Golgi, unraveling the secretory pathway and its relevance to health and disease.
Endoplasmic Reticulum: The endoplasmic reticulum is crucial for protein synthesis and folding. Organelle omics studies elucidate the ER's role in protein quality control, unfolded protein response, and its implications in neurodegenerative diseases.
Centrosome: Centrosomes organize the microtubule network and play a key role in cell division. Organelle omics explores centrosomal proteins, shedding light on cell cycle regulation and centrosome-related disorders.
Extracellular Vesicles: Beyond exosomes, various extracellular vesicles contribute to cell communication. Organelle omics provides a holistic view of the diverse vesicle cargo, elucidating their roles in health, disease, and therapeutic applications.
Organelle Omics Analysis Techniques
Proteomic analysis within organelle omics involves the identification and quantification of proteins present in specific cellular compartments. Cutting-edge mass spectrometry (MS) techniques, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS), enable the high-throughput analysis of the organelle proteome. This approach not only identifies the proteins within an organelle but also provides information about their post-translational modifications, structural conformations, and interactions with other biomolecules. Additionally, advanced quantitative proteomics methods, like isobaric labeling (e.g., TMT and iTRAQ), facilitate the comparison of protein expression levels across different organelles or under varying cellular conditions. The integration of proteomic data contributes to a more detailed understanding of the functional roles and regulatory networks associated with specific organelles.
Metabolomic analysis in organelle omics focuses on unraveling the small-molecule metabolites within cellular compartments. Techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy and high-resolution mass spectrometry (HR-MS) are employed to identify and quantify metabolites, providing insights into the metabolic pathways and biochemical processes occurring within specific organelles. Metabolomics sheds light on the dynamics of energy metabolism, nutrient utilization, and the production of signaling molecules within organelles. Moreover, stable isotope labeling techniques, such as stable isotope-resolved metabolomics (SIRM), enable the tracking of metabolite fluxes, allowing researchers to elucidate the metabolic flux within and between organelles. The integration of metabolomic data enhances our understanding of the metabolic regulation and crosstalk between different organelles, providing a more comprehensive view of cellular homeostasis.
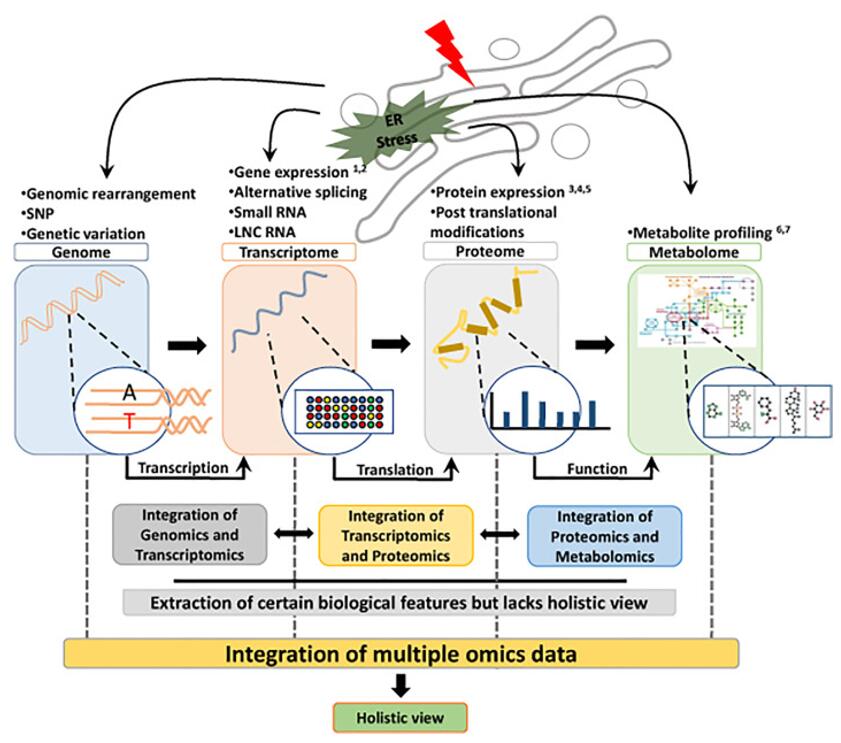
Integration of multi-omics data to gain a holistic overview (Mallick et al., 2022).
Single-Cell Organelle Omics:
Advancements in single-cell technologies allow for the analysis of organelles at the individual cell level. Single-cell genomics, transcriptomics, and single-cell proteomics enable researchers to explore the heterogeneity of organelles within a cell population, uncovering subtle variations and functional differences.
Live-Cell Imaging Techniques:
Live-cell imaging provides dynamic insights into organelle behavior and interactions. Techniques like fluorescence microscopy and confocal imaging allow researchers to visualize organelles in real-time, observing their movement, morphology, and response to stimuli.
Multi-Omics Integration:
Integration of data from multiple omics layers is a crucial aspect of organelle omics analysis. Bioinformatics tools and systems biology approaches facilitate the amalgamation of genomics, transcriptomics, proteomics, and metabolomics data, providing a comprehensive understanding of the interconnected molecular networks within organelles.
Applications of Organelle Omics
Organelle omics, with its multidimensional approach, unfolds a myriad of applications across various domains in cellular biology, biomedicine, and biotechnology.
Disease Research:
Organelle omics serves as a powerful tool in understanding the molecular underpinnings of various diseases. Investigating organelle-specific changes provides critical insights into the mechanisms underlying pathologies, such as mitochondrial dysfunction in neurodegenerative disorders, endoplasmic reticulum stress in diabetes, and aberrant lysosomal activity in lysosomal storage diseases. The identification of organelle-specific biomarkers enhances early disease detection, prognosis, and the development of targeted therapies.
Drug Discovery and Development:
The detailed characterization of organelles facilitates the identification of potential drug targets and the development of novel therapeutic interventions. By uncovering organelle-specific pathways and dysregulations associated with diseases, researchers can design drugs that selectively modulate the functions of specific organelles. Organelle omics also plays a crucial role in drug toxicity studies and the optimization of drug delivery systems, ensuring targeted and effective treatments.
Biotechnological Applications:
In biotechnology, organelle omics contributes to the optimization of cellular processes for enhanced production of biofuels, pharmaceuticals, and valuable biochemicals. By understanding and engineering the functions of organelles, researchers can improve the efficiency of cellular factories, leading to increased yields and cost-effective bioproduction. This has broad implications for sustainable and industrial applications.
Precision Medicine:
Organelle omics provides a foundation for personalized and precision medicine approaches. Understanding the organelle-level variations in individuals allows for targeted therapeutic strategies tailored to specific patients. This is particularly relevant in cancer treatment, where organelle-specific mutations and alterations can guide the selection of precise therapeutic interventions, minimizing side effects and improving treatment outcomes.
Environmental and Evolutionary Studies:
Analyzing organelles across different species contributes to our understanding of evolutionary processes and adaptations. Comparative organelle omics studies reveal conserved and divergent features, shedding light on the evolution of cellular compartments. Additionally, the impact of environmental factors on organelle function can be explored, providing insights into cellular responses to changing ecological conditions.
Functional Genomics and Systems Biology:
Organelle omics is instrumental in advancing functional genomics, allowing researchers to decipher the roles of specific organelles in cellular processes. Integrating data from multiple omics layers facilitates systems biology approaches, enabling the construction of comprehensive models that capture the dynamic interactions and regulatory networks within and between organelles.
Reference
Mallick, Priyanka, et al. "Role of systems biology and multi-omics analyses in delineating spatial interconnectivity and temporal dynamicity of ER stress mediated cellular responses." Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1869.4 (2022): 119210.