Cancer represents a formidable challenge in the realm of biomedical research due to its inherent complexity. At its core, cancer is characterized by uncontrolled cell growth and the acquisition of various genetic, epigenetic, and molecular alterations. These alterations manifest in intricate and heterogeneous patterns across different types of cancer, contributing to the diverse clinical presentations observed in patients. The intricacy of cancer biology demands a nuanced and sophisticated approach to unravel its underlying mechanisms.
To navigate this intricate landscape effectively, advanced analytical techniques have become indispensable. Conventional methods often fall short in providing a comprehensive understanding of the multitude of molecular factors that drive cancer initiation, progression, and metastasis. Advanced analytical techniques, with their high sensitivity and specificity, offer a more detailed and nuanced view of the molecular intricacies associated with cancer. These techniques allow researchers to examine the genomic, proteomic, metabolomic, and transcriptomic landscapes simultaneously, providing a holistic perspective on the molecular events underpinning cancer development.
Omics technologies, in particular, have emerged as powerful tools in dissecting the complex molecular networks involved in cancer. The term "omics" encompasses genomics, proteomics, metabolomics, and transcriptomics, each offering a unique lens through which to explore different facets of the disease. Genomic analysis unveil alterations in the DNA sequence, while proteomic studies shed light on the dynamic protein landscape. Metabolomics provides insights into the metabolic signatures associated with cancer, and transcriptomics elucidates gene expression patterns and regulatory mechanisms. The integration of these omics approaches facilitates a more comprehensive understanding of the molecular underpinnings of cancer, transcending the limitations of individual analysis.
Omics Technologies for Cancer Research:
Omics technologies have revolutionized cancer research by providing comprehensive insights into the molecular intricacies of this complex disease. The term "omics" encompasses various high-throughput approaches that enable the simultaneous analysis of large-scale biological components, such as genes, proteins, metabolites, and transcripts. These technologies play a pivotal role in advancing our understanding of cancer biology, identifying potential biomarkers, and paving the way for personalized medicine.
Genomics:
Cancer Gene Sequencing: Genomic analyses unveil mutations, copy number variations, and structural alterations in cancer-related genes. Identifying these genomic aberrations is crucial for understanding cancer initiation and progression.
Protein Gel and Imaging: Visualization of proteins aids in identifying differentially expressed proteins.
Protein Identification: Utilizing mass spectrometry to identify and quantify proteins associated with cancer.
Quantification and Post-Translational Modification Analysis: Understanding protein abundance and modifications provide insights into cellular processes.
Top Down Proteomics: Analyzing intact proteins offers a holistic view of the proteome.
Protein-Protein Interaction Networks: Uncovering interactions critical for cancer pathways.
Subcellular Proteomics: Examining proteins within specific cellular compartments.
Peptidomics Analysis: Studying cancer-associated peptides.
Peptide Characterization: Analyzing purity and structure-activity relationships of peptides.
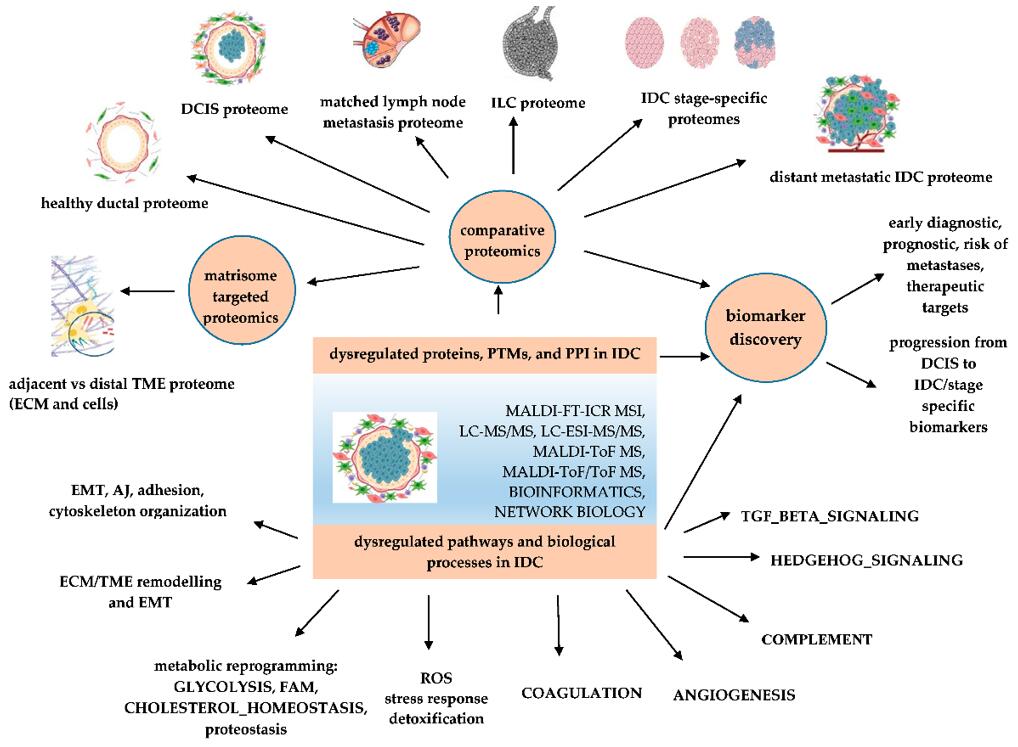
Applications of proteomics-based identification of dysregulated pathways and biomarker discovery in IDC (Neagu et al., 2023).
Untargeted and Targeted Metabolomics: Profiling metabolites in an unbiased or targeted manner.
Metabolic Flux Analysis (MFA): Studying the dynamics of metabolic pathways in cancer cells.
Identification of Unknown Metabolites and Xenobiotic Metabolites: Unraveling novel metabolites associated with cancer.
Lipidomics Analysis: Examining lipid profiles, including untargeted, targeted, exosome lipidomics, and MALDI-imaging.
Transcriptomics:
Gene Chip Technology Services: Investigating gene expression patterns in cancer cells.
RNA Sequencing: Profiling the entire transcriptome for insights into gene expression.
LC-MS and tRNA Sequencing: Exploring RNA modifications.
microRNA Sequencing, Ribo-seq, PCR Chip Technology: Understanding small RNA and regulatory mechanisms.
Bioinformatics Support:
Analyzing and interpreting data from omics experiments: Bioinformatics tools are essential for processing and extracting meaningful information from large omics datasets.
Integration of bioinformatics:
Combining data from genomics, proteomics, and metabolomics for a comprehensive understanding of cancer biology.
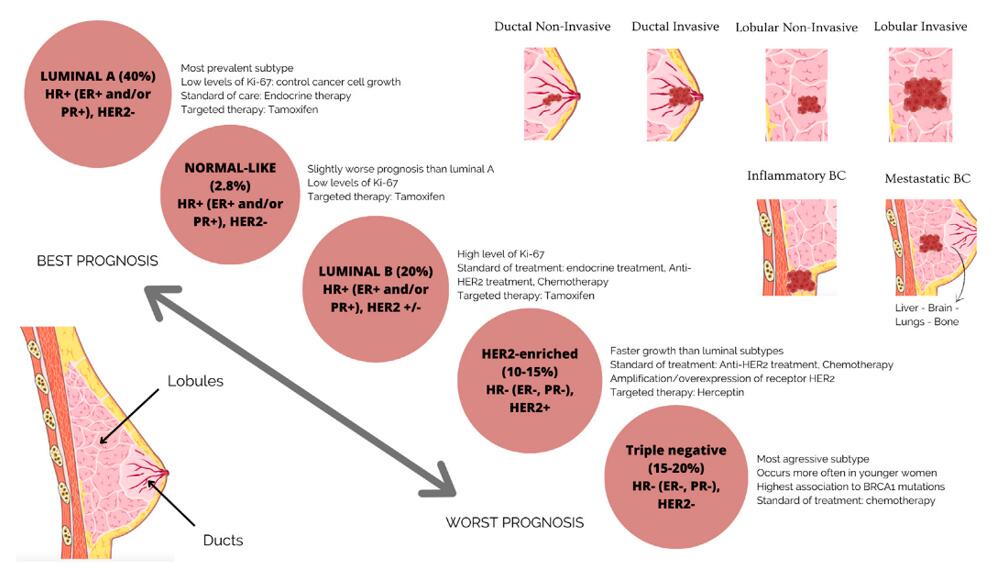
BC subgroups are based on molecular and histological characteristics (Orsini et al., 2023).
Applications in Cancer Research
Omics technologies, particularly genomics and proteomics, play a pivotal role in identifying molecular markers indicative of cancer. These biomarkers can be utilized for early detection, prognosis, and monitoring treatment response.
Early Detection and Diagnosis:
Genomic and proteomic profiling enable the identification of specific genetic mutations, altered protein expression, or circulating biomarkers that can serve as early indicators of cancer. This facilitates timely intervention and improved patient outcomes.
Personalized Medicine Approaches:
The wealth of information provided by omics technologies allows for the development of personalized medicine strategies. By analyzing the unique genomic, proteomic, and metabolic profiles of individual patients, tailored treatment plans can be designed, optimizing therapeutic efficacy and minimizing side effects.
Drug Target Identification and Validation:
Omics technologies help identify potential therapeutic targets by revealing key genes, proteins, or metabolic pathways involved in cancer. These targets can be further validated for their relevance in cancer progression and targeted drug development.
Understanding Tumor Heterogeneity:
The heterogeneity of tumors poses a significant challenge in cancer treatment. Omics technologies enable the characterization of intra-tumor heterogeneity, providing insights into the diverse molecular subtypes within a single cancer type. This information is crucial for developing targeted therapies that address the specific molecular alterations present in individual patients.
Monitoring Treatment Response:
Omics technologies, particularly imaging and liquid biopsy techniques, allow for real-time monitoring of treatment response. Changes in genomic, proteomic, or metabolic profiles can be assessed to gauge the effectiveness of therapeutic interventions and make necessary adjustments to treatment plans.
Identification of Resistance Mechanisms:
Resistance to cancer therapies is a common challenge. Omics technologies help uncover the molecular mechanisms underlying resistance, offering opportunities to develop strategies to overcome treatment resistance and enhance treatment durability.
Exploring Metabolic Signatures:
Metabolomics provides insights into the altered metabolic pathways in cancer cells. Understanding the metabolic signatures associated with different types of cancer contributes to the identification of novel therapeutic targets and the development of metabolism-targeted therapies.
Discovery of Novel Therapeutic Targets:
Integrating data from genomics, proteomics, and metabolomics facilitates the discovery of previously unknown therapeutic targets. This opens avenues for the development of innovative drugs and treatment modalities.
Advancing Immunotherapy:
Omics technologies contribute to the understanding of the tumor microenvironment, immune response, and immunosuppressive mechanisms. This knowledge aids in the development of immunotherapies that harness the patient's immune system to combat cancer.
References
Neagu, Anca-Narcisa, et al. "Proteomics-Based Identification of Dysregulated Proteins and Biomarker Discovery in Invasive Ductal Carcinoma, the Most Common Breast Cancer Subtype." Proteomes 11.2 (2023): 13.
Orsini, Arianna, Chiara Diquigiovanni, and Elena Bonora. "Omics Technologies Improving Breast Cancer Research and Diagnostics." International Journal of Molecular Sciences 24.16 (2023): 12690.