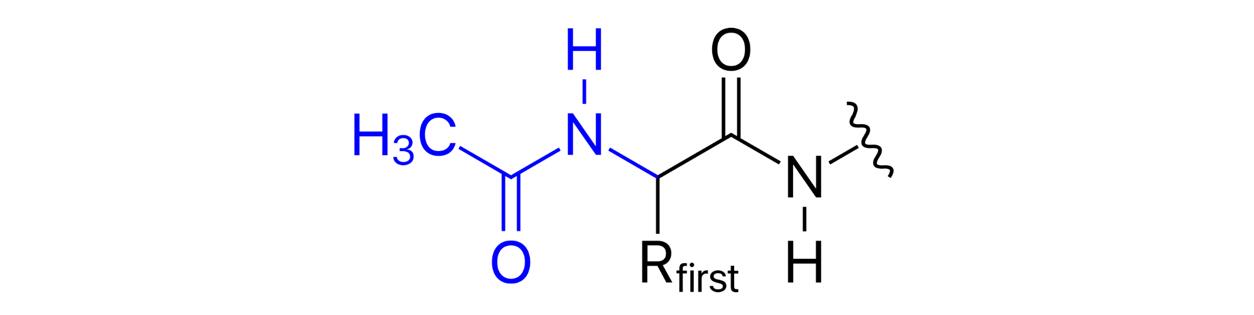
What is N-Acetylation?
The n -acetylation is a highly complex biochemical reaction that transfers the acetyl group (-COCH3) to the nitrogen atom of the amino group in the molecule. This process is commonly referred to as acetylation.
The acetyl group is the central molecule of the reaction and is derived from acetyl coenzyme a (acetyl- coa). Acetyl coenzyme a is an important molecule involved in various metabolic processes, such as the breakdown of carbohydrates, fatty acids and amino acids. It is involved in energy production and the synthesis of many biomolecules.
Enzymes known as acetyltransferases are responsible for catalyzing the transfer of acetyl groups to the nitrogen atom of amino groups. These enzymes are essential in the regulation of many biological processes, including gene expression, protein function and cellular signaling.
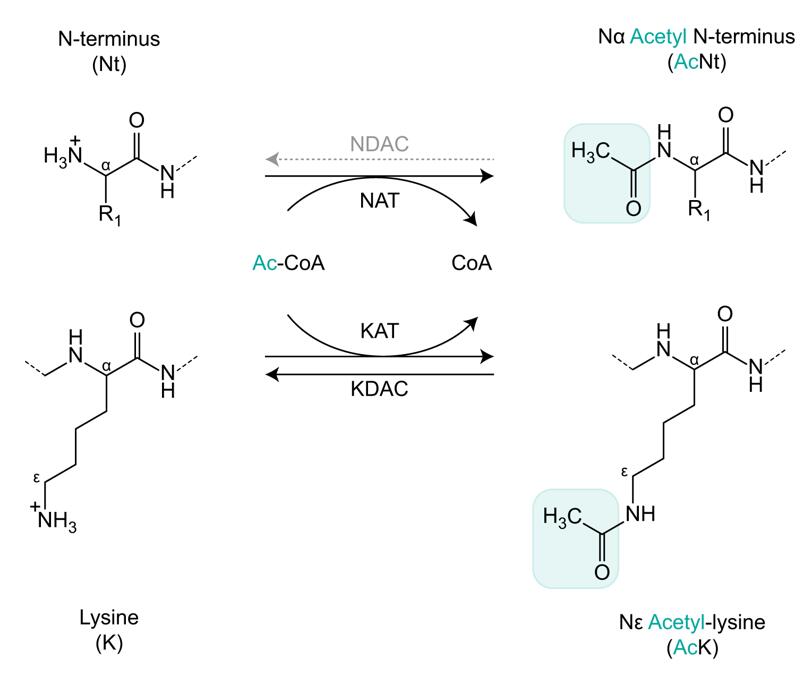
Schematic outline of N-terminal and lysine protein acetylation (Ree et al., 2018).
What is an N-Acetyl Group?
The N-acetyl group, a functional moiety arising from the acetylation of an amino nitrogen, bears noteworthy structural features. It comprises a carbonyl group (-C=O), covalently attached to a methyl group (-CH3), both of which are tethered to the nitrogen atom. The molecular formula of the N-acetyl group, embodying the elemental constituents of carbon, hydrogen, nitrogen, and oxygen, is denoted as C2H5NO.
What is the Purpose of N-Terminal Acetylation?
The intricate process of N-terminal acetylation, a subtype of N-acetylation, entails the transference of an acetyl group to the amino group at the N-terminus of a protein or peptide. This post-translational modification assumes critical functions in protein biology. For instance, it furnishes the protein with a robust shield against proteolytic degradation, thereby endowing it with increased stability. Furthermore, N-terminal acetylation augments protein-protein interactions, amplifying the chances of protein complex formation.
Another crucial role of N-terminal acetylation is linked to the intricate mechanisms of protein trafficking, cellular signaling, and gene regulation. Its involvement in these processes underscores its significance in cellular biology.
What is N-Acetylation in Post-Translational Modification?
Post-translational modification (PTM) refers to the modification of proteins after they have been synthesized. N-Acetylation is a common form of PTM that occurs in many different proteins and peptides.
N-Acetylation can have various effects on protein function, stability, and localization. It can also regulate protein-protein interactions and modulate enzymatic activity. In some cases, N-acetylation is essential for protein function, while in other cases, it is dispensable.
What is the N-Acetyl Amino Acid?
A prominent category of amino acids, N-acetyl amino acids, are subject to a transformative process through the attachment of an N-acetyl group. Amongst these modified amino acids, N-acetyl cysteine (NAC) - arising from the amino acid cysteine - remains the most prevalent.
The addition of an acetyl moiety to amino acids can exert diverse effects on their functions and characteristics. For instance, acetylation of lysine residues present in histone proteins assumes a crucial role in governing gene expression. Conversely, acetylation of other amino acids may impact protein folding, stability, and localization. Such alterations can trigger significant changes in protein structure and behavior.
What Drugs are Metabolized by N-Acetylation?
In the realm of drug metabolism, N-acetylation emerges as a pivotal metabolic pathway for numerous drugs, particularly those containing primary aromatic amines or hydrazine moieties. Prominent instances of drugs that undergo N-acetylation comprise isoniazid, procainamide, and hydralazine.
The intricate process of N-acetylation can yield both active and inactive metabolites, contingent on the specific drug and the involved enzyme. For instance, N-acetylation of isoniazid can generate both an active metabolite (acetylisoniazid) and an inactive metabolite (N-acetylhydrazine). This transformation of drugs can exert significant impacts on their therapeutic efficacy and safety profiles.
How to Analyze N-Acetylation?
Liquid chromatography-mass spectrometry (LC-MS) has emerged as one of the most prevalent methodologies for analyzing N-acetylation. This technique involves the separation and detection of N-acetylated peptides and proteins based on their mass-to-charge ratio (m/z) and retention time.
To conduct LC-MS analysis of N-acetylation, samples are usually subjected to protease digestion, such as trypsin, to generate peptides. The resulting peptides are then segregated using reverse-phase liquid chromatography (RPLC) based on their hydrophobicity.
Following this, electrospray ionization (ESI) is employed to ionize the segregated peptides before they are introduced into the mass spectrometer. The mass spectrometer gauges the mass-to-charge ratio (m/z) of the peptides and produces a mass spectrum, which can be employed to detect N-acetylated peptides based on their characteristic mass shifts.
The quantification of N-acetylation levels can also be accomplished through LC-MS, utilizing isotope labeling methodologies such as stable isotope labeling with amino acids in cell culture (SILAC) or isobaric tags for relative and absolute quantification (iTRAQ).
The LC-MS analysis of N-acetylation holds the potential to offer valuable insights into the sites and levels of N-acetylation in intricate biological samples, and can serve as an influential tool for studying protein function and activity regulation.
At Creative Proteomics, our experienced team of scientists and state-of-the-art facilities possess extensive expertise in LC-MS analysis of N-acetylation and other post-translational modifications. Our services provide high-quality, reliable data, which can aid in advancing your research objectives.
Reference
Ree, Rasmus, Sylvia Varland, and Thomas Arnesen. "Spotlight on protein N-terminal acetylation." Experimental & molecular medicine 50.7 (2018): 1-13.