Understanding the Importance of Glycosylation in Protein Function
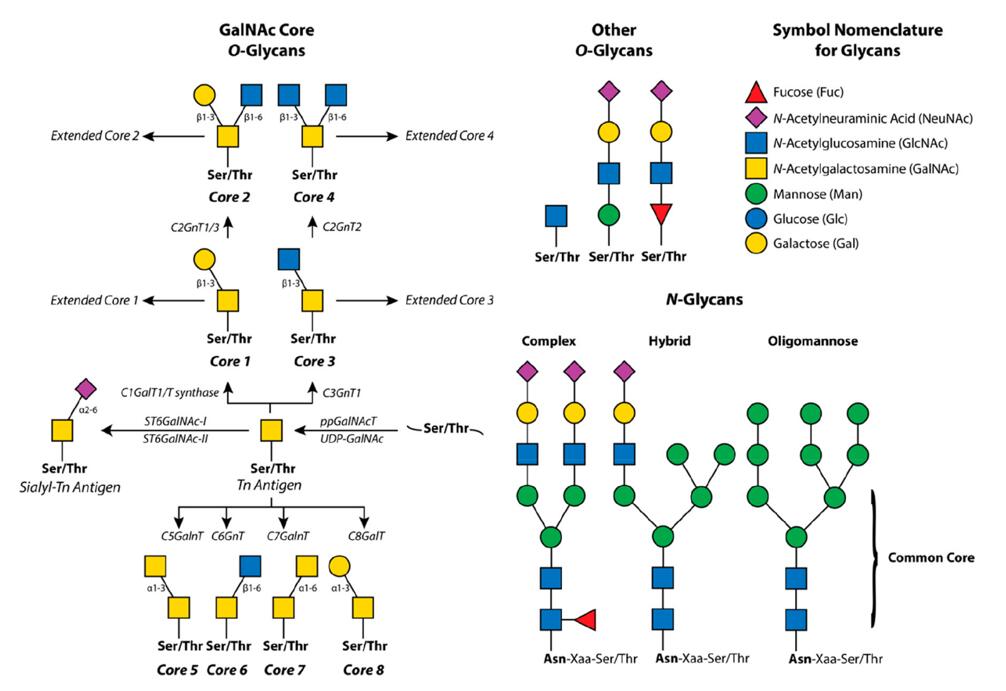
Glycosylation is a common post-translational modification that plays a critical role in the function of many proteins. It involves the covalent attachment of carbohydrate molecules, called glycans, to specific amino acid residues on the protein backbone. The glycans can affect protein folding, stability, and activity, as well as interactions with other molecules. O-glycosylation is a specific type of glycosylation where the glycans are attached to the hydroxyl group of serine or threonine residues. In this article, we will discuss the importance of O-glycan analysis in understanding the function of glycosylated proteins.
Glycosylation Modification
The dexterity of glycosylation manifests in two distinct methods: N-glycosylation and O-glycosylation. The former pertains to the ligation of glycans to the nitrogen atom of the asparagine residue, while the latter involves the binding of glycans to the hydroxyl group of serine or threonine residues. In comparison to N-glycans, O-glycans are more abbreviated and diversified in their structure. These minuscule compounds can comprise a myriad of monosaccharides and form links in diverse ways, producing a plethora of possible configurations.
Techniques for Studying Glycosylated Proteins
The intricacy and diversity of glycans can present a formidable challenge when studying glycosylated proteins. Nevertheless, several techniques are available to facilitate glycoprotein analysis. One such technique is mass spectrometry (MS), a potent tool for identifying and quantifying glycans and glycopeptides. MS facilitates the determination of the molecular weight, composition, and linkage of glycans, which are vital pieces of information for comprehending the characteristics and functionality of glycoproteins. Another valuable approach for glycan analysis is high-performance liquid chromatography (HPLC). This technique separates and analyzes glycans based on their physical and chemical properties, providing an opportunity to assess the heterogeneity of glycans. Glycan microarrays are another formidable weapon in the arsenal of glycan structure research. These microarrays enable the examination of a wide range of glycans for their binding affinity to a protein of interest, enabling researchers to map the glycan fingerprinting of glycosylated proteins with a high-throughput approach.
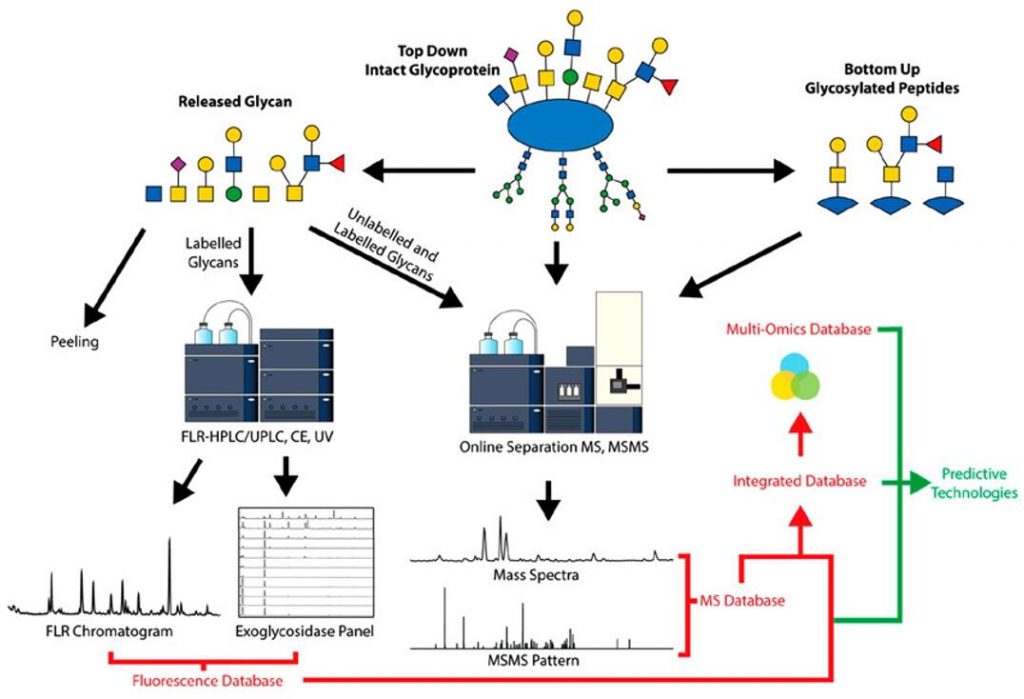
Methods for the Characterization of O-Glycans (Wilkinson et al., 2020)
Glycan Structure Research
The configuration of glycans holds a paramount significance in their function and interplay with other molecules. The intricate nature of glycan branching and fucosylation plays a pivotal role in shaping the binding affinity of a protein to its ligand. Moreover, the sialylation of N-glycans can exert a notable influence on the half-life of a protein in the bloodstream. Therefore, a comprehensive understanding of the structure of glycans is indispensable for uncovering the multifarious functional implications of glycosylation. Glycan structure research encompasses a gamut of analytical techniques, such as mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, which facilitate the determination of the structure of glycans. These cutting-edge techniques enable researchers to study the intricate molecular structure of glycans, identify the linkages between the monosaccharides, and elucidate the three-dimensional shape of these complex molecules. With the help of these sophisticated techniques, scientists can establish a more comprehensive understanding of how the structure of glycans influences glycoprotein function and interactions, providing crucial insights for further glycosylation modification studies.
Profiling Glycosylation Patterns in Health and Disease
Glycan fingerprinting, a technique for profiling glycosylation patterns of proteins in a sample, has proven to be an effective tool in this pursuit. This technique involves the enzymatic release of glycans from the protein backbone, followed by MS or HPLC analysis of the released glycans to identify changes in glycosylation patterns. By comparing the glycan profiles of different biological samples, such as healthy and diseased tissues or different stages of development, researchers can identify key differences that may provide insight into disease mechanisms or potential biomarkers.
High-Throughput Glycomics: Accelerating Glycosylation Research
The use of high-throughput glycomics has revolutionized the study of glycosylation patterns by accelerating research and increasing the scale of analysis. Automated and high-throughput techniques allow the rapid screening of a large number of samples, making it possible to identify changes in glycosylation patterns efficiently. This approach has been widely used in drug development and glycobiology research, allowing researchers to screen for potential drug targets based on changes in glycosylation patterns in diseased tissues. High-throughput glycomics has also been instrumental in studying the role of glycosylation in cell signaling and other biological processes.
Challenges and Advances of O-Glycosylation Detection
The convoluted and intricate study of O-glycosylation in both health and disease, cannot be overstated. The multifaceted implications of aberrant O-glycosylation are far-reaching and have been definitively linked to a multitude of conditions, including cancer and inflammatory disorders. In cancer, it has been demonstrated that changes in O-glycosylation have the capacity to imperil cell adhesion and migration, ultimately resulting in the ghastly advancement and metastasis of tumors. Similarly, in inflammatory disorders like rheumatoid arthritis, changes in O-glycosylation of proteins involved in immune regulation have been reported. Consequently, the understanding of the intricate O-glycosylation patterns in diverse biological samples, serves as a pivotal aspect of identifying potential biomarkers and therapeutic targets. With further headway in O-glycosylation research, the path to personalized medicine and precision therapies can become more evident.
Applications of O-Glycan Analysis
The analysis of O-glycans is an integral aspect of comprehending the intricate role of glycosylation in multifarious biological processes. The connection between changes in O-glycosylation and the advancement of numerous diseases, such as cancer and inflammatory disorders, has been well-documented. The meticulous analysis of O-glycans can provide an intricate understanding of the underlying mechanisms of these diseases, thereby facilitating the development of innovative therapeutic strategies. Furthermore, O-glycan analysis can be leveraged to delve into the evolution of glycosylation across a gamut of species and gain insights into the diversification of glycosylation in various organisms. In totality, the immense potential of O-glycan analysis in advancing our understanding of glycosylation and its multifaceted role in health and disease cannot be understated.
Reference
Wilkinson, Hayden, and Radka Saldova. "Current methods for the characterization of O-glycans." Journal of proteome research 19.10 (2020): 3890-3905.