Phosphoproteomics analysis is a powerful tool used to investigate the phosphorylation of proteins in complex biological systems. It provides insights into the regulation of cellular signaling pathways and can be used to identify novel phosphorylation sites and regulatory networks. Creative Proteomics is a leading provider of phosphoproteomics analysis services, utilizing a range of advanced technologies to offer comprehensive analysis of phosphorylated proteins. In this article, we will explore the methods and instruments used for phosphoproteomics analysis, as well as the characteristics and advantages of these approaches.
Methods and Instruments Used for Phosphoproteomics Analysis
The primary method used in phosphoproteomics analysis is mass spectrometry (MS). There are several MS-based approaches that can be used to analyze phosphorylated proteins, including bottom-up, middle-down, and top-down approaches. Bottom-up proteomics involves the digestion of proteins into peptides, followed by the separation of phosphopeptides from non-phosphorylated peptides. The phosphopeptides are then analyzed using MS. Middle-down proteomics involves the analysis of intact proteins or protein fragments, while top-down proteomics involves the analysis of intact proteins.
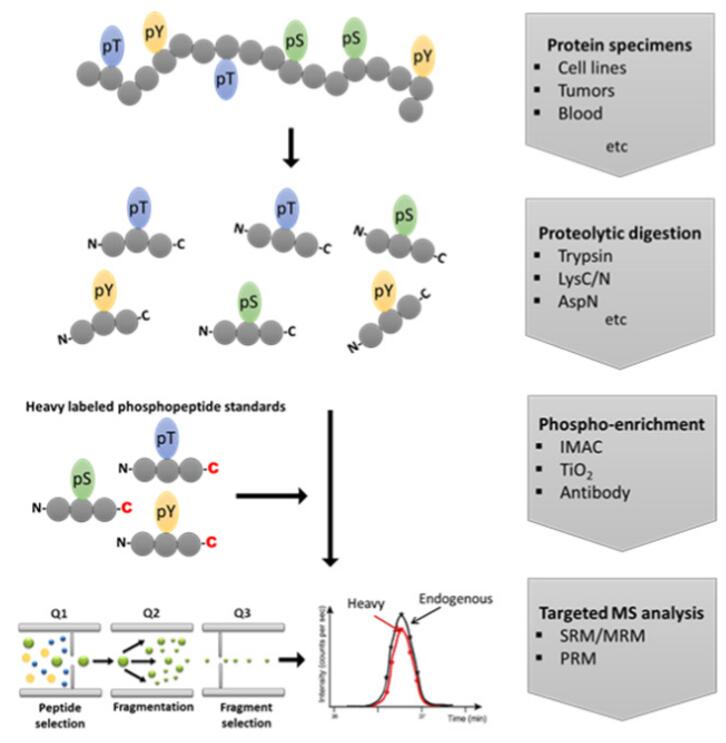
Standard workflow for targeted analysis of protein phosphorylation[1]
To enrich for phosphopeptides, a range of techniques can be used, including titanium dioxide (TiO2) chromatography, immobilized metal affinity chromatography (IMAC), and phospho-specific antibodies. TiO2 and IMAC are both metal ion-based methods that selectively bind to phosphorylated peptides, while phospho-specific antibodies recognize and bind to phosphorylated proteins or peptides.
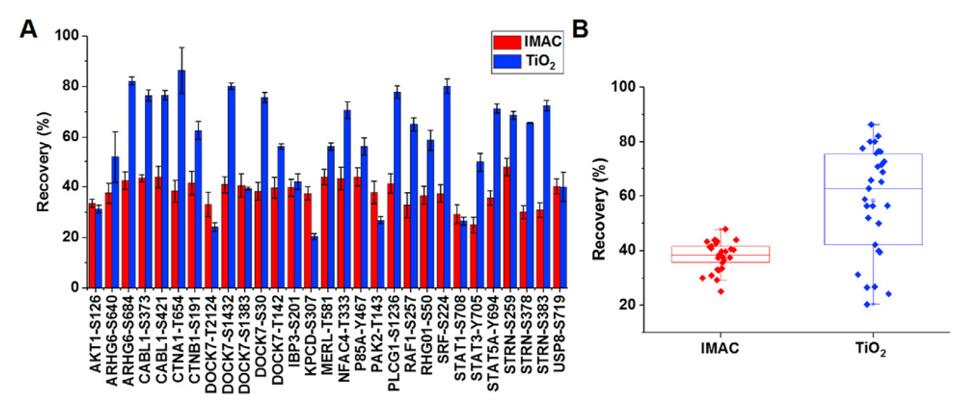
Recoveries of IMAC and TiO2 enrichment of individual phosphopeptides (A) and overall box plot (B)[2]
Application, Characteristics, and Advantages of Phosphoproteomics Analysis
Phosphoproteomics analysis has several applications, including the identification of novel phosphorylation sites, the characterization of phosphorylation-based signaling networks, and the analysis of changes in protein phosphorylation in response to stimuli. The characteristics of these methods include high sensitivity, accuracy, and specificity, enabling the detection and quantification of thousands of phosphorylated proteins in a single experiment.
One of the key advantages of phosphoproteomics analysis is its ability to provide a comprehensive view of the phosphorylation state of a biological system. This can lead to a better understanding of cellular signaling pathways and the identification of potential therapeutic targets. Additionally, phosphoproteomics analysis can be used to study changes in protein phosphorylation in response to drug treatments or environmental changes, providing valuable insights into the mechanisms of action of drugs and potential biomarkers of disease.
Phosphoproteomics analysis is a powerful approach that allows for the comprehensive study of the phosphorylation state of proteins in complex biological systems. Utilizing a range of advanced technologies and techniques, Creative Proteomics offers comprehensive phosphoproteomics analysis services for researchers in the life sciences. With the ability to identify and quantify thousands of phosphorylated proteins in a single experiment, phosphoproteomics analysis has the potential to revolutionize our understanding of cellular signaling pathways and provide new insights into the mechanisms of disease.
Reference
[1] Dakup, P. P., Feng, S., et al. (2023). Targeted Quantification of Protein Phosphorylation and Its Contributions towards Mathematical Modeling of Signaling Pathways. Molecules, 28(3), 1143.
[2] Urban, J. (2022). A review on recent trends in the phosphoproteomics workflow. From sample preparation to data analysis. Analytica Chimica Acta, 1199, 338857.