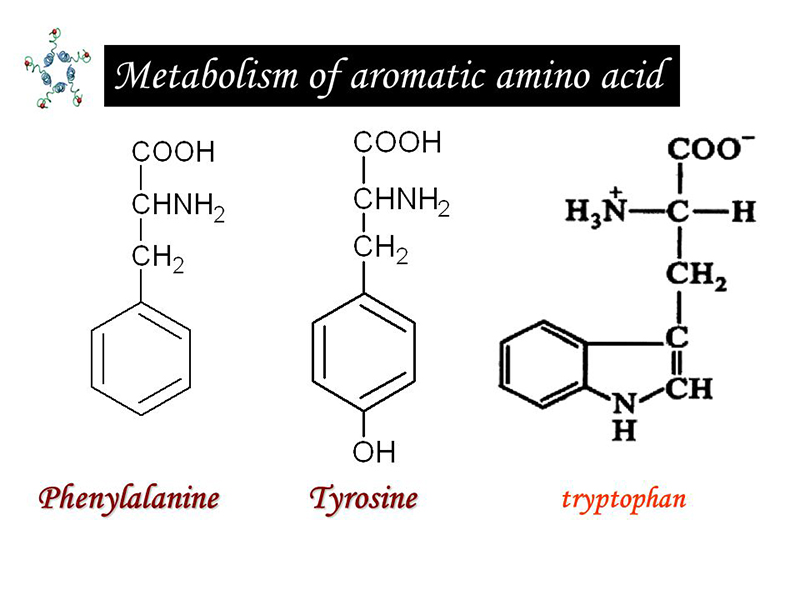
Aromatic amino acids include phenylalanine, tyrosine, and tryptophan, of which phenylalanine and tyrosine have a similar structure, and phenylalanine can be converted into tyrosinase. That’s why we will combine this two together to discuss.
Phenylalanine and tyrosine
A. Phenylalanine is generally converted to tyrosine in vivo
Completing by the phenylalanine hydroxylase catalytic introduction of hydroxyl, which coenzyme is the tetrahydro Bioperine. The reaction produces dihydrobiopterin, being catalyzed by dihydrofolate reductase with NADPH + H reduced to tetrahydro. Phenylalanine hydroxylase catalyzed the reaction is irreversible, the body cannot be converted to phenylalanine threonine.
B. Synthesis of catecholamines and melanin
Tyrosine is catalyzed by tyrosine hydroxylase which is also tetrahydrobiopterin as coenzyme plus monooxygenase to produce 3,4ahydrohydroxyphenylalanine L-DOPA, and is catalyzed by dopa decarboxylase to form dopamine. Dopamine in the dopamine β-oxidase catalyzed by hydroxylation of β carbon atoms, resulting in norepinephrine. And then produce epinephrine with norepinephrine methylation that is methylated by SAM. Dopamine, norepinephrine, adrenaline collectively referred to as catecholamine. Tyrosine hydroxylase is the rate-limiting enzyme of catecholamine synthesis, which is regulated by the feedback of the final product.
In melanocytes, tyrosine can form dopa with the catalyzation of tyrosinase, and dopa is oxidized to form dopaquinone that generating into the pathways of synthesizing melanin. The resulting dopaquinone is further cyclized and decarboxylated to produce indole quinone which will facilitate melanin synthesis.
C. Tyrosine is raw sugar and ketone amino acid tyrosine by transamination action to produce p-hydroxyphenyl pyruvate, further decomposition of acetylene and fumaric acid
D. Dysbolism
It is known that there are many Dysbolism in phenylalanine and tyrosine metabolism. Especially for the phenylketonuria (PKV) which is due to the lack of phenylalanine hydroxylase. Phenylalanine cannot be properly converted to tyrosine, but accumulation in vivo, producing phenylpyruvate by the role of transamination (part of the reduction to phenylacetic acid) and excreted from the urine. The accumulation of phenylpyruvate is toxicity on the central nervous system that will lead to the disease associated with mental retardation. We can control the content of phenylalanine in the diet which will be helpful for the intelligence development if we can detect at the early stage.
Tryptophan is an essential amino acid. But the amount of content is small in most proteins, less human body intake and less decomposition. In addition to participating in protein synthesis, it can produce 5 serotonin by oxidative decarboxylation. And it can be degraded to produce raw sugar, ketogenic ingredients, generating a carbon unit and niacin etc. In the process.
A. Tryptophan decomposition
The pyrene ring was first opened by tryptophan-2,3-dioxygenase to form N-Formylkynurenine. Under the action of formamidase, formylated kynurenine deformyl formate formic acid and kynurenine. Formic acid can participate in a carbon unit metabolism while kynurenine has three different metabolic directions:
. Urethrins mainly catalyze the production of 3-hydroxy kynurenine by kynurenine-3-monoxygenase, followed by kynureninase (with PLP as coenzyme ) Catalyzed hydrolysis of alanine, and forming 3-hydroxyanthranilate. Alanine can be converted to pyruvate by transamination while 3-hydroxybenzoic acid can generate α-ketoacetic acid by oxidation cracking, decarboxylation, and other reaction. Thus, tryptophan is a raw sugar and ketone amino acid.
A small amount of kynurenine can produce canine uric acid by transamination and condensation.
A small amount of alanine is produced after cleavage of alanine
B. The production of tryptophan
Catalytic cracking of 3-hydroxyanthranilate-3,4-dioxygenase in tryptophan catabolism can generate niacin which is the key component of NAD (P) +.
All the above information is about the metabolism of Aromatic amino acid.
Related service: