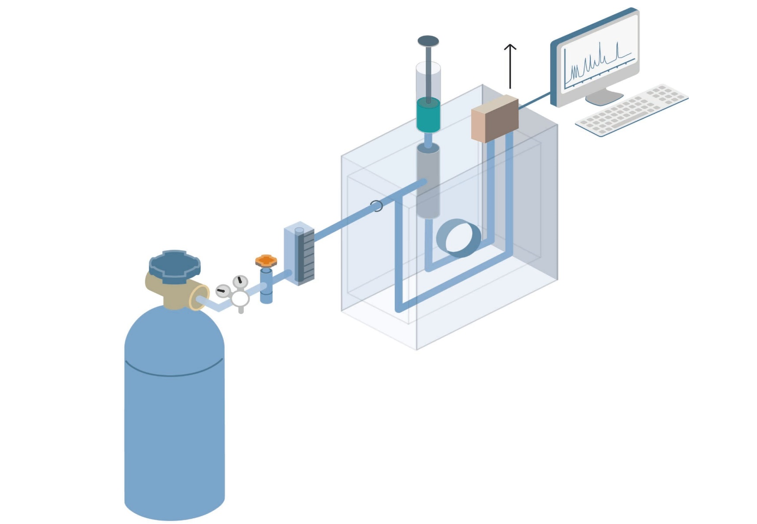
What is gas chromatography?
Chromatography is a technique that separates components in a mixture by the difference in partitioning behavior between mobile and stationary phases. Gas chromatography (GC) is one of the popular chromatography techniques to separate volatile compounds or substances. The mobile phase is a gas such as helium, and the stationary phase is a high-boiling liquid that is adsorbed on a solid. Because of its simplicity, high sensitivity, and the ability to effectively separate mixtures, gas chromatography has become one of the most important tools in proteomics, metabolomics and lipidomics.
The principle of gas chromatography
Components in the mixture are distributed between two phases, one of which is a stationary phase, and the other is a mobile phase gas, or carrier gas, that carries the mixture through the stationary phase. Compounds in the mobile phase interact with the stationary phase as they pass through. Due to the differences in properties and structures of each component, the size and affinity of each interaction with the stationary phase are different. Therefore, under the same driving force, the retention time of different components differs in the column, thus moving out of the column in different orders.
Advantages of gas chromatography
1) High separation efficiency and analysis speed: for example, gasoline samples can obtain more than 200 chromatographic peaks in 2 hrs. A general sample analysis can be completed in 20 minutes.
2) Small sample consumption and high detection sensitivity: 1 ml of gas sample consumption, 0.1 µl of liquid sample consumption, a few mg of solid sample consumption. Proper detectors can detect impurities in the tens to a few parts per million.
3) Gas chromatography has good selectivity and can be used to analyze azeotropic mixtures and samples with close boiling points. For example, some isotopes, cis-trans isomers, adjacent or intertrans isomers, optical isomers, etc.
4) Wide range of applications, although mainly used to analyze gases and volatile organic substances;under certain conditions, it can also be used to analyze high boiling point substances and solid samples.
Disadvantages of gas chromatography
It can only be used to analyze volatile substances. Some highly polar substances can be derived to increase their volatility for GC analysis, but the process can be complex and may introduce errors in quantitative analysis.
How does gas chromatography work?
First, the sample is introduced into a stream of inert gas, or a carrier, which is usually helium or argon. For a liquid sample, it needs to be evaporated before being injected into the carrier. Sample components move through the packed column at a rate affected by the degree of interaction of each component with the stationary non-volatile phase. Substances that interact more with the stationary phase are delayed and thus separated from substances that interact less. When the components are eluted from the column, they can be quantified and/or collected through the detector for further analysis.
References:
- McNair H M, Miller J M, Snow N H. Basic gas chromatography. John Wiley & Sons, 2019.
- Scott R P W. Introduction to Analytical Gas Chromatography, Revised and Expanded. CRC press, 2017.