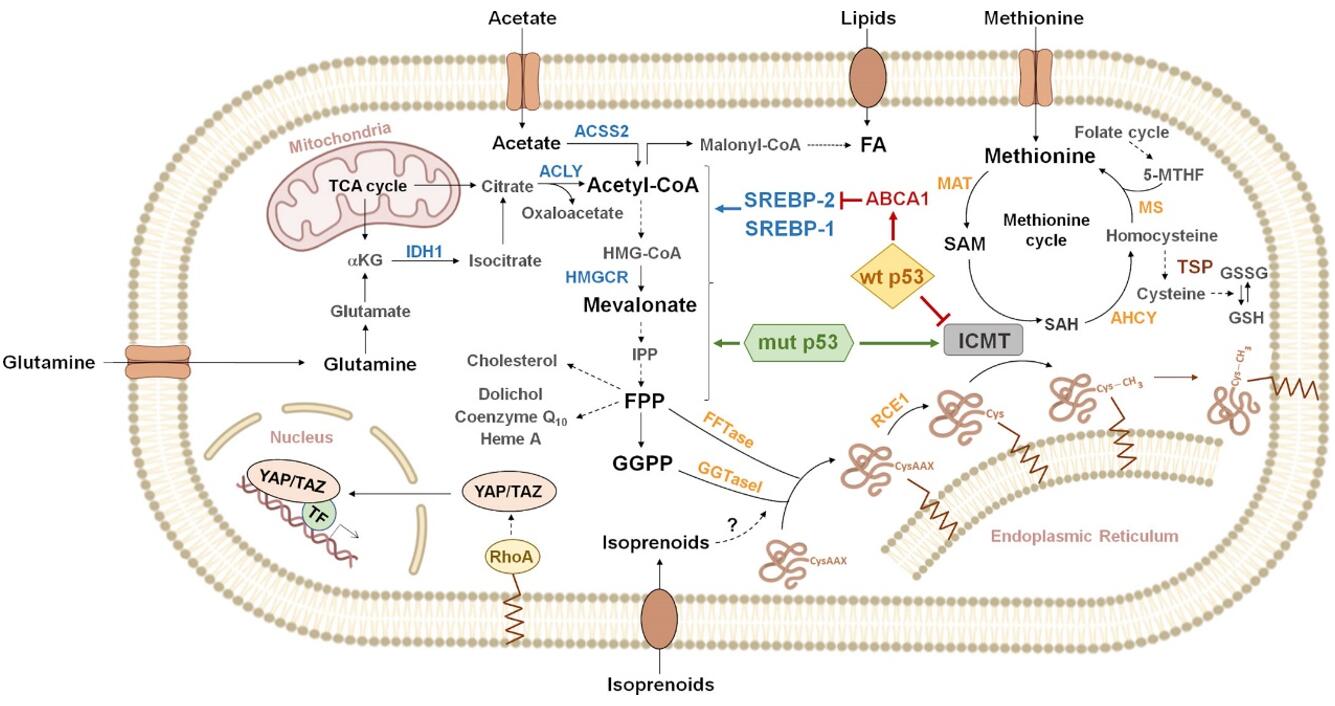
The Genesis of Mevalonate Pathway: Origins and Reactions
The Mevalonate Pathway, a cornerstone of cellular metabolism, begins with the conversion of acetyl-CoA to acetoacetyl-CoA. This crucial metabolic transformation marks the initiation of a series of enzymatic reactions that ultimately lead to the synthesis of mevalonate. Acetyl-CoA, a key intermediate in various metabolic pathways including glycolysis, fatty acid oxidation, and amino acid metabolism, serves as the primary substrate for this initial step.
The conversion of acetyl-CoA to acetoacetyl-CoA is catalyzed by the enzyme acetyl-CoA acetyltransferase, also known as thiolase. This enzyme facilitates the condensation of two molecules of acetyl-CoA, resulting in the formation of acetoacetyl-CoA. This reaction represents the first committed step in the mevalonate pathway, setting the stage for subsequent transformations.
Acetoacetyl-CoA, the product of this condensation reaction, serves as the precursor for further elongation and modification within the pathway. Through a series of enzymatic steps involving the sequential addition of additional acetyl-CoA units, reduction, and phosphorylation reactions, acetoacetyl-CoA undergoes progressive transformations to ultimately yield mevalonate, the central intermediate of the pathway.
Overall, the conversion of acetyl-CoA to acetoacetyl-CoA represents the crucial starting point of the mevalonate pathway, initiating a cascade of reactions that culminate in the synthesis of mevalonate. This process underscores the intricate nature of cellular metabolism, where small molecules are intricately orchestrated to generate essential building blocks for cellular function and organismal health.
Essential Products of the Mevalonate Pathway
In the intricate landscape of cellular metabolism, the mevalonate pathway stands as a pivotal route for the synthesis of essential molecules crucial for cellular function and organismal health. At the heart of this pathway lie its indispensable products, each playing a vital role in diverse physiological processes.
One of the primary products of the mevalonate pathway is cholesterol, a fundamental component of cell membranes and a precursor for the synthesis of steroid hormones, bile acids, and vitamin D. Cholesterol serves as a structural lipid that regulates membrane fluidity and permeability, influencing cell signaling and receptor function. Additionally, cholesterol plays a crucial role in the formation of lipid rafts, specialized microdomains within the membrane that facilitate protein trafficking and signal transduction.
Beyond its role in membrane structure and function, cholesterol serves as a precursor for the biosynthesis of steroid hormones, including cortisol, aldosterone, estrogen, and testosterone. These hormones play diverse roles in regulating metabolism, immune function, reproduction, and stress response, highlighting the importance of cholesterol in maintaining physiological homeostasis.
Another significant product of the mevalonate pathway is the class of molecules known as isoprenoids, which encompass a diverse array of compounds with varied biological functions. Isoprenoids serve as essential building blocks for molecules involved in cell signaling, protein modification, and antioxidant defense.
Among the key isoprenoids synthesized via the mevalonate pathway are farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which serve as lipid anchors for the post-translational modification of proteins known as prenylation. Prenylated proteins play critical roles in intracellular signaling pathways, membrane trafficking, and cytoskeletal organization. Additionally, isoprenoids contribute to the biosynthesis of molecules such as dolichols, ubiquinone (coenzyme Q), and heme A, which are essential for processes including protein glycosylation, electron transport chain function, and oxygen transport.
Furthermore, the mevalonate pathway generates other important isoprenoid-derived molecules, such as sterols, carotenoids, and quinones, each with distinct roles in cellular physiology. Sterols, including cholesterol and ergosterol, contribute to membrane integrity and serve as precursors for steroid hormones and bile acids. Carotenoids function as antioxidants and play a role in photoprotection and vision. Quinones, such as ubiquinone and plastoquinone, participate in electron transport chain reactions, facilitating ATP production and energy metabolism.
Regulation of the Mevalonate Pathway
Overview of Regulatory Mechanisms
The regulation of the mevalonate pathway occurs at multiple levels, including transcriptional control, post-translational modifications, and feedback inhibition. These regulatory mechanisms collectively modulate the activity of key enzymes in response to cellular needs and environmental cues, ensuring adaptive metabolic responses.
Transcriptional Control: One of the primary modes of regulation of the mevalonate pathway is through the transcriptional regulation of key enzymes. This process is mediated by transcription factors that bind to specific DNA sequences in the promoter regions of target genes, thereby modulating their expression levels. Sterol regulatory element-binding proteins (SREBPs) are master regulators of cholesterol and lipid metabolism and play a central role in the transcriptional regulation of genes encoding enzymes involved in the mevalonate pathway. SREBPs are activated in response to low cellular cholesterol levels, leading to the upregulation of mevalonate pathway genes and subsequent cholesterol synthesis.
Post-Translational Modifications: Post-translational modifications, such as phosphorylation, acetylation, and ubiquitination, also play important roles in regulating the activity of enzymes within the mevalonate pathway. Phosphorylation, mediated by protein kinases, is a common regulatory mechanism that can either activate or inhibit enzyme activity. For example, HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway, is subject to phosphorylation by multiple kinases, including AMP-activated protein kinase (AMPK) and protein kinase A (PKA), which regulate its activity in response to cellular energy status and signaling pathways.
Feedback Inhibition: Feedback inhibition is another critical mechanism by which the mevalonate pathway is regulated. Accumulation of downstream metabolites, such as cholesterol and isoprenoids, serves as allosteric inhibitors of key enzymes within the pathway, thereby attenuating their activity and preventing excessive flux through the pathway. HMG-CoA reductase, for example, is allosterically inhibited by cholesterol, its end product, as well as by other downstream metabolites such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). This feedback inhibition helps to maintain cholesterol homeostasis and prevent overproduction of isoprenoids.
Regulation of HMG-CoA Reductase
HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway, is a key target for regulation due to its central role in cholesterol biosynthesis. The activity of HMG-CoA reductase is tightly controlled through a variety of mechanisms, including transcriptional regulation, post-translational modifications, and feedback inhibition.
Transcriptional Regulation: The expression of the gene encoding HMG-CoA reductase is regulated by multiple transcription factors, including SREBPs and liver X receptors (LXRs), which respond to cellular cholesterol levels and metabolic signals. SREBPs promote the transcription of HMG-CoA reductase and other mevalonate pathway genes under conditions of low cellular cholesterol, while LXRs induce the expression of genes involved in cholesterol efflux and catabolism.
Post-Translational Modifications: HMG-CoA reductase activity is also subject to regulation through post-translational modifications, including phosphorylation, ubiquitination, and proteolytic cleavage. Phosphorylation of HMG-CoA reductase by kinases such as AMPK and PKA inhibits its activity, while dephosphorylation by phosphatases relieves this inhibition. Additionally, ubiquitination targets HMG-CoA reductase for degradation via the ubiquitin-proteasome pathway, providing another level of regulation.
Feedback Inhibition: Feedback inhibition by cholesterol and other downstream metabolites is a crucial mechanism for regulating HMG-CoA reductase activity. Elevated levels of cholesterol and isoprenoids allosterically inhibit HMG-CoA reductase, reducing its enzymatic activity and suppressing cholesterol biosynthesis. This feedback inhibition helps to maintain cholesterol homeostasis and prevent excessive accumulation of cholesterol and its derivatives.
Impact of Feedback Inhibition on the Mevalonate Pathway
Feedback inhibition plays a critical role in modulating the activity of enzymes within the mevalonate pathway and ensuring metabolic homeostasis. Accumulation of downstream metabolites, such as cholesterol and isoprenoids, serves as allosteric inhibitors of key enzymes, effectively attenuating their activity and preventing excessive flux through the pathway.
In addition to HMG-CoA reductase, other enzymes within the mevalonate pathway are subject to feedback inhibition by downstream metabolites. For example, enzymes involved in the synthesis of isoprenoids, such as farnesyl pyrophosphate synthase and geranylgeranyl pyrophosphate synthase, are allosterically inhibited by their respective end products, FPP and GGPP. This feedback inhibition helps to regulate the production of isoprenoids and maintain cellular homeostasis.
Overall, the regulation of the mevalonate pathway is a complex and finely tuned process that involves multiple levels of control, including transcriptional regulation, post-translational modifications, and feedback inhibition. These regulatory mechanisms ensure the precise control of metabolite flux through the pathway, allowing cells to adapt to changing metabolic demands and maintain physiological homeostasis. Understanding the regulation of the mevalonate pathway provides valuable insights into its physiological significance and its role in human health and disease.
Impact of the Mevalonate Pathway on Human Health:
The mevalonate pathway plays a critical role in human health, exerting influence over a wide range of physiological processes. Dysregulation of this pathway can have profound implications for human health, contributing to the pathogenesis of various diseases.
Cholesterol Synthesis and Cardiovascular Health: One of the most well-known associations with the mevalonate pathway is its role in cholesterol biosynthesis. Cholesterol is essential for the structural integrity of cell membranes and serves as a precursor for the synthesis of steroid hormones and bile acids. However, excessive accumulation of cholesterol, particularly low-density lipoprotein (LDL) cholesterol, in the bloodstream is a major risk factor for cardiovascular diseases such as atherosclerosis, coronary artery disease, and stroke. Inhibition of the mevalonate pathway through the use of statin drugs, which target the enzyme HMG-CoA reductase, has been a cornerstone of cholesterol-lowering therapy and has been shown to reduce the risk of cardiovascular events and mortality.
Isoprenoids and Cellular Signaling: Isoprenoids derived from the mevalonate pathway play crucial roles in cellular signaling and protein prenylation. Prenylated proteins, such as small GTPases and nuclear lamins, are involved in a wide range of cellular processes, including cell growth, differentiation, and apoptosis. Dysregulation of protein prenylation has been implicated in the pathogenesis of cancer, neurodegenerative diseases, and inflammatory disorders. Targeting the mevalonate pathway to modulate protein prenylation has emerged as a potential therapeutic strategy for these conditions.
Immune Function and Inflammatory Diseases: The mevalonate pathway also plays a role in immune function and inflammation. Isoprenoids generated by this pathway are essential for the activation and function of immune cells, including T cells, B cells, and macrophages. Dysregulation of the mevalonate pathway has been implicated in autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, as well as autoinflammatory disorders such as mevalonate kinase deficiency (MKD) and hyperimmunoglobulin D syndrome (HIDS).
In summary, the mevalonate pathway has far-reaching implications for human health, impacting cholesterol metabolism, cellular signaling, immune function, and inflammatory responses. Understanding the role of this pathway in health and disease provides valuable insights into potential therapeutic targets and strategies for the prevention and treatment of various disorders.
Analytical Methods for Mevalonate Pathway Metabolites:
Analyzing metabolites of the mevalonate pathway is essential for understanding its physiological roles and dysregulation in disease states. Various analytical techniques have been developed for the quantification and characterization of mevalonate pathway metabolites, providing valuable tools for basic research and clinical diagnostics.
Liquid Chromatography-Mass Spectrometry (LC-MS): LC-MS is a powerful analytical technique that combines the separation capabilities of liquid chromatography with the detection and identification capabilities of mass spectrometry. LC-MS allows for the sensitive and specific quantification of mevalonate pathway metabolites, including cholesterol, isoprenoids, and their derivatives, in biological samples such as serum, plasma, and tissue extracts. This technique enables researchers to investigate changes in metabolite levels associated with disease states and therapeutic interventions.
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is another widely used analytical technique for the analysis of mevalonate pathway metabolites. GC-MS separates metabolites based on their volatility and allows for the identification and quantification of compounds such as cholesterol, fatty acids, and sterols. GC-MS is particularly useful for analyzing volatile metabolites and is commonly used in metabolomics studies to profile cellular metabolism and identify biomarkers of disease.
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy is a non-destructive analytical technique that provides information about the structure and dynamics of molecules in solution. NMR can be used to quantify mevalonate pathway metabolites such as cholesterol, isoprenoids, and their derivatives, as well as to study their interactions with proteins and other biomolecules. NMR spectroscopy is valuable for elucidating the biochemical mechanisms underlying mevalonate pathway regulation and its role in health and disease.
Reference
Etichetti, Carla M. Borini, et al. "Beyond the mevalonate pathway: control of post-prenylation processing by mutant p53." Frontiers in Oncology 10 (2020).