During the completion of the human genome, a wide variety of additional "omics" technologies arose, such as transcriptomics. Peptidomics represents a short version of "peptide proteomics". Since the high diversity of peptides in living systems and their involvement in key regulatory processes, there is a need for improved peptide discovery. The peptidomics means the comprehensive visualization and analysis of small polypeptides, thus covering the mass range between proteomics and metabonomics. There is no clear definition to distinguish between peptides and proteins. The most common description is that peptides range from two amino acids (dipeptides) to small proteins with a molecular mass of 20 kDa. There are large numbers of peptides in human body fluids and have many possible functionalities.
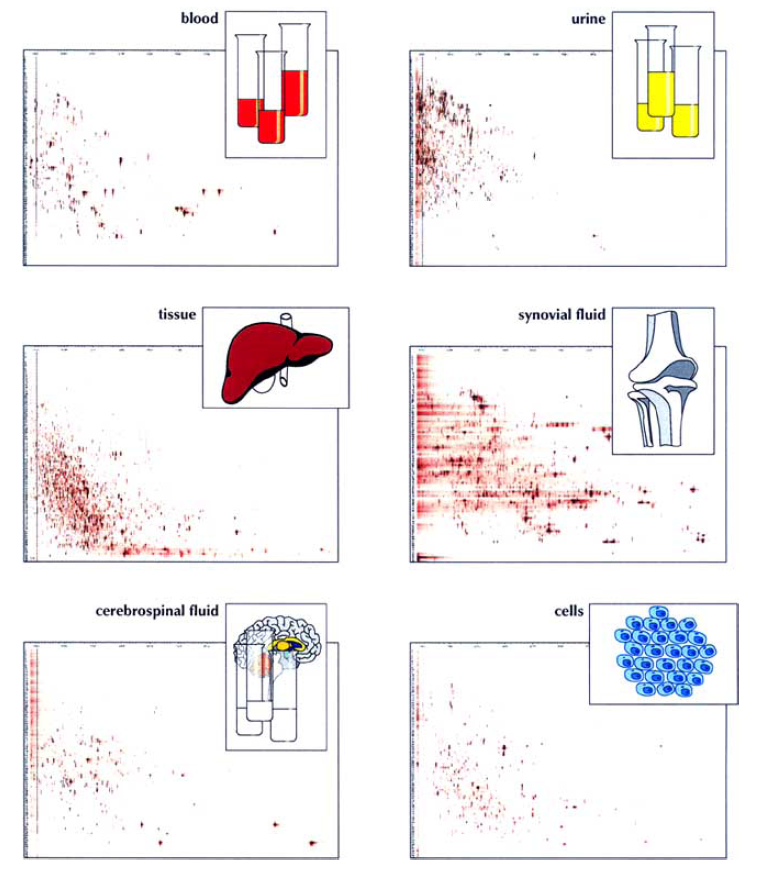
Figure 1. Peptidomes from body fluids, tissue, and cells. (Schulz-Knappe P, et al., 2005)
Peptidomics in Biomarkers
Peptidomics can be used for biomarker discovery. The data from a biomarker can be applied to diagnostic implications, molecular and cellular events of the pathology, and new therapeutic approaches. Many peptidomics studies are searching for molecular analytes which correlate with disease states, including C-peptide of proinsulin in diabetes, natriuretic peptides in heart diseases or amyloid peptides correlated with Alzheimer's. Peptide biomarkers have to fulfill two tasks, including qualitative analysis and quantitative analysis, which will allow for the selection of those peptides that are valuable for the desired drug development process. Qualitative analysis means to show which peptides are present in a sample while quantitative analysis means to discover the concentration.
Peptidomics in drug targets
Currently, there are mainly four types of molecules developed for the treatment of human diseases, including small molecules, antibodies, peptides, and proteins. With the developments in several technologies in the areas of peptide synthesis, screening, stability, and modifications, peptides are now recognized as lead molecules for therapeutics. In addition, they have some features that make them ideal drug targets. These peptide properties include chemical and biological diversity, high specificity, affinity and potency, as well as unique 3D characteristics. Since the no accumulation in organs, peptides display a low toxicity at therapeutic doses, and are less immunogenic than antibodies. However, there are some drawbacks. They are low stability in body fluids and the difficulty in transporting them to target organs.
There are more than 67 peptide drugs are currently on the market. Some examples of the therapeutic potential of endogenous peptides include adrenocorticotropic hormone (ACTH), β-amyloid peptides (A β), calcitonin, desmopressin, incretin mimetics, insulin, leuprolide acetate, oxytocin, and pramlintide acetate.
Technologies of Peptidomics
The study of endogenous peptides is often compromised by protein fragments during conventional sample handling. In peptidomics studies, it’s important to control proteolysis, not only to avoid peptide degradation but also mainly to prevent contaminating the peptide sample with proteolytic fragments of larger proteins from the same source. Peptides analysis needs preparation and analysis methods that are substantially different from those typically used for proteins.
The peptides have some characteristics, including the peptide size and charge state, which complicate the analysis of endogenous peptides. The endogenous peptides range in size from 2 to over 100 amino acids. In addition, the charge state of the endogenous peptides is not nearly as uniform as for tryptic peptides. And because of the blocked N-terminus and the absence of lysine, some endogenous peptides have no positive charge. However, the goal of most peptidomics studies is to identify all of the abundant peptides, including some peptides with positive charges or large sizes. Because peptides vary in multiple aspects, multiple peptide purification methods can be used sequentially on the same sample. But this strategy has some drawbacks, like limiting the reproducibility of the method and increasing sample preparation time.
With the development of electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), mass spectrometry has become an ideal tool for peptide analysis. These soft ionization methods can ionize intact peptide molecules and acceleration into a vacuum without substantial fragmentation. Various mass analyzers have applied to peptidomics, including orbitraps, quadrupole time-of-flight (Q-TOF), and micro-TOF. In addition, tandem mass spectrometry is required for additional information for sequencing. There are some commonly used fragmentation techniques for peptidomics, including collision-induced dissociation (CID), electron transfer dissociation (ETD), and electron-capture dissociation (ECD).
The peptides have a great potential as biomarkers and important drug targets for therapies. Peptidomics is used as an ideal tool for discovery and validation of biomarkers, drug targets, and lead candidates. Equipped with professional high resolution, Creative Proteomics provides the professional peptidomics services, including:
References:
- Schrader M, Schulz-Knappe P, Fricker L D. Historical perspective of peptidomics. EuPA Open Proteomics, 2014, 3: 171-182.
- Clynen E, Baggerman G, Husson S J, et al. Peptidomics in drug research. Expert opinion on drug discovery, 2008, 3(4): 425-440.
- Schulz-Knappe P, Schrader M, Zucht H D. The peptidomics concept[J]. Combinatorial chemistry & high throughput screening, 2005, 8(8): 697-704.