A disulfide bond is a special post-translational modification (PTM), which is a covalent linkage between two cysteine residues. It forms through oxidation of thiol (–SH) groups. This bond is denoted as –S–S–. Disulfide bonds serve as molecular "braces." They stabilize protein tertiary and quaternary structures. They also guide correct folding. In secretory and extracellular proteins, these linkages are especially prevalent.
Proteins that traverse the secretory pathway often acquire disulfide bonds in the endoplasmic reticulum. Cytosolic proteins, by contrast, rarely contain these linkages due to the reducing environment. Secreted enzymes, antibodies, and growth factors commonly rely on disulfide bonds. These bonds resist thermal denaturation. They also resist proteolytic cleavage.
Chemical Properties of Disulfide Bonds in Proteins
Thermodynamics & Redox Potential of –S–S– Linkages
The thermodynamic stability of a disulfide bond depends on the redox potential of the two cysteine thiols. The standard redox potential for the cystine/cysteine pair is approximately –250 mV. A more positive cellular redox potential favors disulfide formation. A more negative potential promotes bond reduction. Disulfide bonds confer roughly 60 kJ/mol of stabilization energy under physiological conditions.
pKa of Cysteine Thiols and Its Impact on Bond Formation
The pKa of a free cysteine thiol is about 8.3. In a protein microenvironment, this pKa can shift from 5.5 to 9.0. A lower pKa increases the fraction of thiolate anion at physiological pH. Thiolate is the reactive species that attacks another thiol to form disulfide. Local electrostatics, hydrogen bonding, and nearby charged residues modulate this pKa.
Influence of Local Environment on Bond Stability
Hydrophobic pockets shield disulfide bonds from solvent. This shielding enhances bond stability. Polar environments, by contrast, can facilitate bond exchange and isomerization. Nearby metal ions or cofactors may also perturb redox potential. Conformational strain around the bond can lower the activation barrier for reduction.
Mechanisms of Disulfide Bond Formation and Isomerization
Enzymatic Catalysis by Protein Disulfide Isomerases (PDI)
Protein disulfide isomerases (PDIs) are oxidoreductases in the endoplasmic reticulum. They possess active-site motifs Cys–Gly–His–Cys (CGHC). The N-terminal thiolate in CGHC attacks a substrate's disulfide to form a mixed-disulfide intermediate. The C-terminal thiol then resolves this intermediate. PDIs also catalyze isomerization of non-native disulfides.
Oxidative Folding Pathways in the Endoplasmic Reticulum
Oxidative folding links disulfide formation to protein folding pathways. ER oxidases such as ERO1 regenerate the oxidized state of PDIs. This regeneration uses oxygen as the ultimate electron acceptor. The cascade ensures a steady supply of oxidizing equivalents. It also prevents over-oxidation of client proteins.
Non-Enzymatic Mechanisms of Disulfide Bond Formation
Non-enzymatic disulfide formation occurs in vitro under controlled redox buffers. Glutathione disulfide (GSSG) acts as a mild oxidant. Diamide and copper(II) catalysts can also promote formation. These chemical approaches mimic the ER environment. They may yield scrambled disulfides without subsequent isomerization steps.
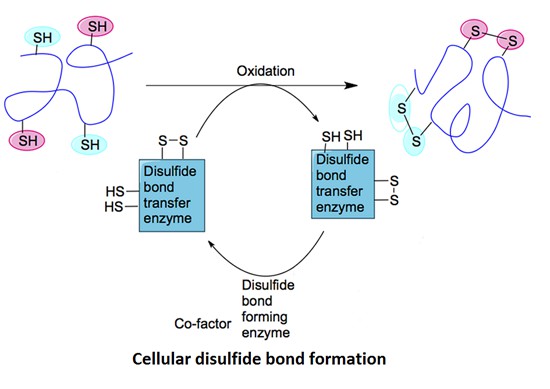 Figure 1. Cellular disulfide bond formation in proteins. (Patil N A, et al., 2015)
Figure 1. Cellular disulfide bond formation in proteins. (Patil N A, et al., 2015)
What is the difference between a disulfide bond and a peptide bond?
| Feature | Disulfide Bond (–S–S–) | Peptide Bond (–CO–NH–) |
|---|---|---|
| Chemical Nature | Covalent linkage between two cysteine sulfur atoms. | Amide linkage between carboxyl and amino groups. |
| Formation Reaction | Oxidation of two thiol (–SH) groups to form S–S. | Condensation (dehydration) reaction releasing H₂O. |
| Bond Type | Redox-sensitive, reversible under reducing conditions. | Stable amide bond; not redox-active. |
| Location in Protein | Between side chains of cysteines (often extracellular or secretory). | Backbone of every protein (links amino acids). |
| Structural Role | Stabilizes tertiary/quaternary fold via cross-links. | Defines primary structure and peptide chain. |
| Stability | Stable in oxidizing environments; reduced in cytosol. | Highly stable under physiological conditions. |
| Typical Environment | Endoplasmic reticulum, extracellular matrix, secretory pathway. | Throughout all cellular compartments. |
| Enzymatic Catalysis | Facilitated by PDIs, QSOX, Ero1, non-enzymatic oxidants. | Ribosome-catalyzed during translation; proteases reverse. |
Which Proteins Have Disulfide Bonds?
Secreted and Extracellular Proteins
Most secretory proteins and plasma proteins possess disulfide bonds to maintain their folded structures in the extracellular matrix. Notable examples include:
- Insulin: A peptide hormone composed of A and B chains connected by two interchain disulfide bonds, with an additional intrachain disulfide in the A-chain.
- Albumin: The most abundant plasma protein, albumin contains 17 disulfide bonds that maintain its globular shape and stability in the circulatory system.
- Immunoglobulins (IgG, IgA, IgM): Disulfide bonds connect heavy and light chains and stabilize the Fc and Fab regions. These bonds are critical for antibody structure, function, and effector mechanisms such as antigen recognition and immune complex formation.
Membrane and Transmembrane Proteins
Several membrane-bound proteins, particularly those on the cell surface, contain disulfide bonds that influence their folding and ligand-binding functions:
- Integrins: These adhesion molecules contain multiple disulfide bonds that regulate conformational states involved in cell-cell and cell-matrix interactions.
- GPCRs (G-protein-coupled receptors): Many members of this large receptor family have extracellular disulfide bridges that stabilize ligand-binding domains and modulate receptor activation.
- Ion channels: Certain ion channels, such as voltage-gated Ca²⁺ channels, include disulfide bonds in their extracellular loops, influencing ion selectivity and gating behavior.
Structural Proteins
Structural proteins rich in cysteine residues often rely on disulfide bridges to form stable networks:
- Keratin: Found in hair, nails, and skin, keratin's rigidity and resilience stem from intermolecular disulfide crosslinks between polypeptide chains.
- Collagen: Although predominantly stabilized by hydrogen bonds, some types of collagen, especially in non-fibrillar forms, also exhibit disulfide bonds near their C-terminal domains for proper trimerization.
- Elastin: Involved in tissue elasticity, elastin precursors form interchain disulfide linkages before crosslinking into mature elastic fibers.
Enzymes and Regulatory Proteins
Enzymes that are secreted or operate in oxidative compartments often contain disulfide bonds that support their activity and structural integrity:
- Ribonuclease A (RNase A): A classical model protein, RNase A contains four disulfide bonds crucial for maintaining catalytic conformation.
- Lysozyme: This antibacterial enzyme has four disulfide bonds stabilizing its compact, globular fold necessary for hydrolytic activity.
- Tissue plasminogen activator (tPA): A serine protease used in clot-dissolution therapies; disulfide bonds maintain its domain architecture essential for fibrin binding.
Viral and Pathogen-Derived Proteins
Viruses and some bacterial exotoxins utilize disulfide bonds to stabilize surface glycoproteins and toxin structures, aiding in host invasion and immune evasion:
- HIV gp120: The HIV envelope glycoprotein is rich in disulfide bonds that support its CD4 receptor-binding site and shield immunodominant regions.
- SARS-CoV-2 spike protein: Contains several disulfide linkages in its receptor-binding domain (RBD), critical for ACE2 receptor interaction and vaccine target stability.
- Diphtheria toxin: Composed of two domains connected by a disulfide bond that must be reduced in the cytosol to release the catalytic subunit.
Intracellular Exceptions
Though rare, some cytosolic or mitochondrial proteins do contain transient or stable disulfide bonds:
- Thioredoxin: A redox-active protein that forms temporary disulfide bonds during catalysis.
- Mitochondrial intermembrane proteins: Some proteins, like Mia40 substrates, form disulfide bonds during import and folding in the mitochondrial intermembrane space, an oxidizing compartment within the otherwise reducing intracellular environment.
Analytical Techniques for Disulfide Bond Characterization
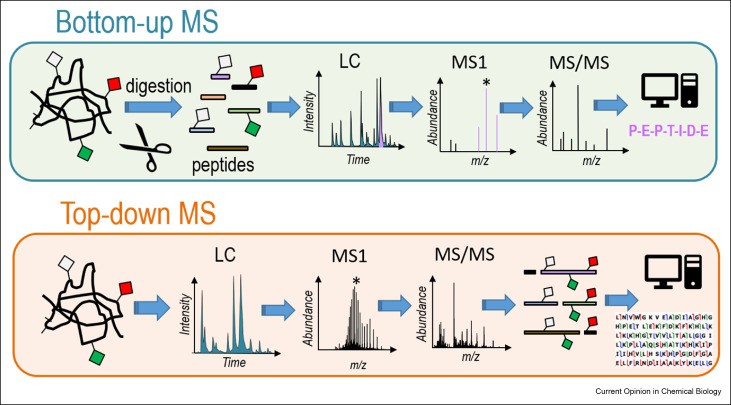
Top-Down and Bottom-Up Proteomics Strategies
Top-down proteomics analyzes intact proteins. It preserves native disulfide architecture. Spectra from high-resolution MS (e.g., Fourier-transform ion cyclotron resonance) reveal disulfide connectivities. Electron capture dissociation (ECD) and electron transfer dissociation (ETD) fragment backbone bonds but leave S–S linkages intact. This permits direct mapping of paired cysteines in a single experiment. Data analysis uses deconvolution algorithms to assign mass shifts corresponding to disulfide-linked fragments.
Bottom-up proteomics begins with proteolysis under non-reducing conditions. Trypsin or chymotrypsin yields peptides that remain linked by disulfide bridges. LC-MS/MS then separates and identifies these crosslinked peptides. Collision-induced dissociation (CID) spectra show characteristic neutral losses of 32 Da. Differential alkylation—labeling free thiols before reduction—distinguishes inter- versus intramolecular disulfides.
Chromatographic Separation of Disulfide-Linked Peptides
Reversed-phase HPLC resolves peptides by hydrophobicity; disulfide bonds increase retention. Gradients of acetonitrile in water with trifluoroacetic acid give sharp peaks. Hydrophilic interaction chromatography (HILIC) exploits the polar nature of cystine loops to enhance separation. Ion-exchange chromatography can fractionate peptides by net charge differences introduced by alkylation reagents. Affinity chromatography uses thiol-reactive resins (e.g., pyridyl disulfide agarose) to enrich disulfide-containing species. Combined techniques improve sensitivity and reduce sample complexity for reliable disulfide mapping.
Applications of Disulfide Bond Engineering in Therapeutic Protein Design
Optimizing Microbial and Mammalian Expression Systems
Recombinant protein production systems, such as Escherichia coli and CHO cells, are frequently engineered to enhance disulfide bond formation. In microbial hosts, oxidative folding is promoted by co-expression of periplasmic oxidases and isomerases. In mammalian cells, modulating ER redox potential or overexpressing PDIs improves folding efficiency and product yield. These strategies are critical for producing disulfide-rich therapeutic proteins at commercial scale.
Engineering Disulfide Bonds to Enhance Protein Stability
Rational disulfide bond engineering is a powerful technique for enhancing protein thermostability, reducing aggregation, and improving shelf-life. By introducing disulfide bridges at strategic positions, researchers can constrain protein dynamics and reduce entropy in the unfolded state. Computational tools and structural databases assist in identifying optimal sites for disulfide insertion without compromising activity or solubility.
Antibody and Biopharmaceutical Development
Disulfide bond engineering has played a transformative role in antibody drug development. For example, stabilizing the Fab and Fc domains with additional disulfide bonds improves pharmacokinetic profiles and formulation stability. In bispecific antibodies, disulfide reshuffling enables selective heterodimerization. Similarly, disulfide-linked payloads in antibody-drug conjugates (ADCs) offer controlled drug release in reducing tumor environments. These advancements underscore the versatility of disulfide chemistry in therapeutic innovation.
Case Analysis of Disulfide Bond
Identification of the Thiol Isomerase-binding Peptide, Mastoparan, as a Novel Inhibitor of Shear-induced Transforming Growth Factor β1 (TGF-β1) Activation
Journal: Journal of Biological Chemistry
Published: 2013
Background
Transforming Growth Factor β1 (TGF-β1) is a disulfide-bonded homodimeric cytokine secreted in an inactive large latent complex (LLC) by platelets and other cells. Shear stress can activate latent TGF-β1, and previous studies have implicated thiol-disulfide exchange in this process. Thiol isomerases like protein disulfide isomerase (PDI) are thought to catalyze these redox changes.
Purpose
The study aimed to investigate the role of thiol isomerases in shear-induced activation of latent TGF-β1 and to evaluate whether mastoparan, a wasp venom peptide known to inhibit PDI, could serve as a novel inhibitor of this activation process.
Methods
- Platelet releasates and recombinant LLC from HEK293T cells were exposed to shear stress or stirring in the presence of mastoparan or control peptide (M17).
- Active and total TGF-β1 were measured by ELISA and a bioassay.
- Free thiol labeling and affinity chromatography using mastoparan-conjugated columns were used to identify interacting proteins.
- Reduced and oxidized PDI were tested for their roles in activating TGF-β1.
- Antibodies against PDI were used to assess the specificity of mastoparan's inhibitory effect.
Results
- Mastoparan inhibited shear- and stirring-induced activation of latent TGF-β1 by 75–90%.
- Affinity chromatography identified several mastoparan-binding proteins including PDI, ERp5, ERp57, ERp72, latent TGF-β1, LAP, and LTBP-1.
- The inhibitory effect of mastoparan was reduced by anti-PDI antibody, suggesting involvement of PDI.
- Reduced PDI (but not oxidized PDI) inhibited TGF-β1 activation, supporting a redox-based mechanism.
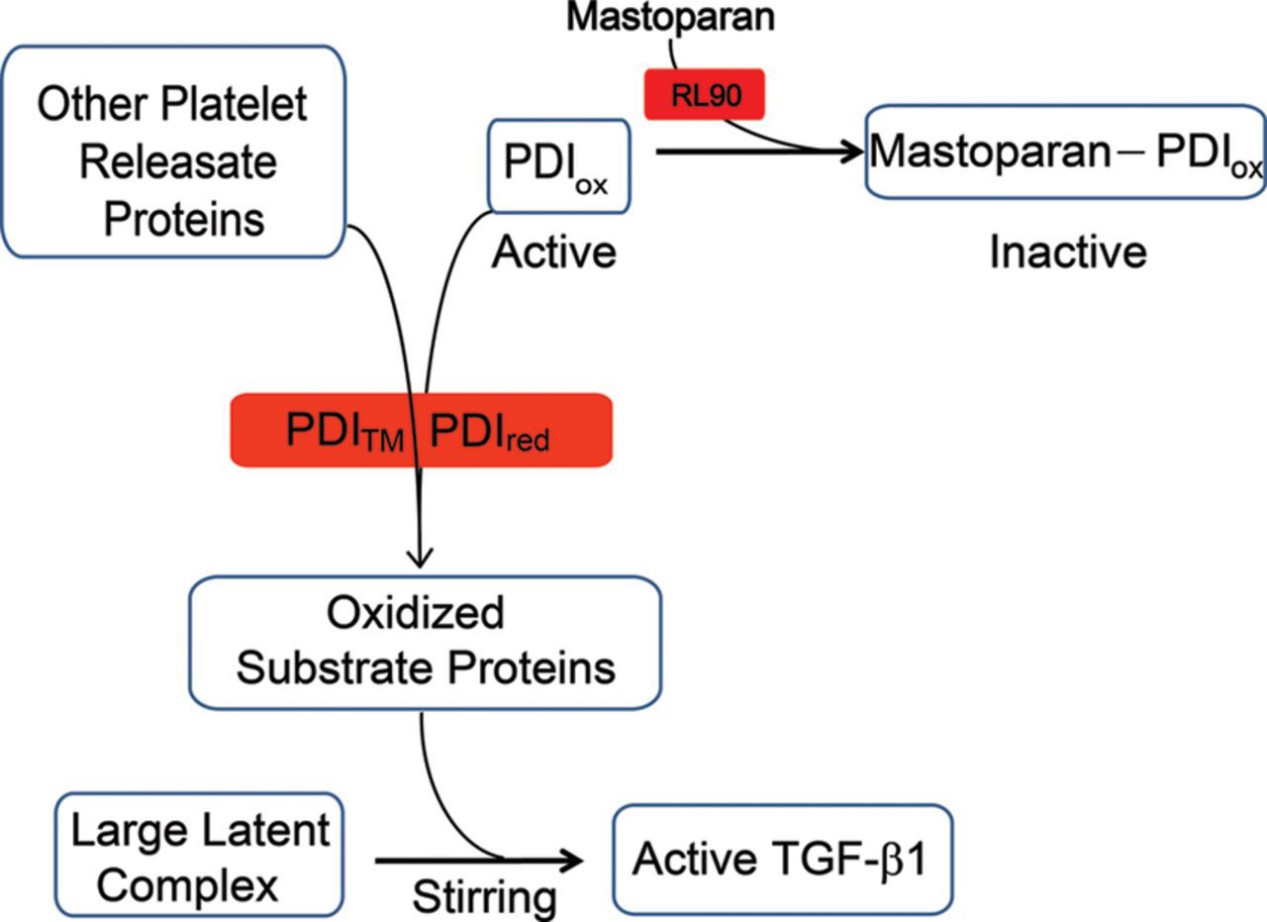 Figure 2. Working model of the thiol-dependent component of shear-induced TGF-β1 activation.
Figure 2. Working model of the thiol-dependent component of shear-induced TGF-β1 activation.
Conclusion
Thiol isomerases, particularly PDI, play a critical role in shear-induced activation of latent TGF-β1 via thiol-disulfide exchange. Mastoparan is a novel inhibitor of this activation pathway and may help identify molecular participants in TGF-β1 regulation.
References
- Bulaj G. Formation of disulfide bonds in proteins and peptides. Biotechnology advances, 2005, 23(1): 87-92. DOI: 10.1016/j.biotechadv.2004.09.002
- Khoo, Keith, and Raymond S. Norton. Role of disulfide bonds in peptide and protein conformation. Amino acids, peptides and proteins in organic chemistry: Analysis and function of amino acids and peptides. Wiley-VCH Verlag GmbH & Co. KGaA, 2011. 395-417. DOI: 10.1002/9783527631841.ch11
- Patil N A, et al. Cellular disulfide bond formation in bioactive peptides and proteins. International journal of molecular sciences, 2015, 16(1): 1791-1805. DOI: 10.3390/ijms16011791
Our products and services are for research use only.