Monoclonal Antibody Absolute Quantification via LC-MS/MS
What is precise quantification of monoclonal antibodies?
Monoclonal antibody (mAb) drugs have become a cornerstone of modern therapeutic strategies, offering targeted treatments for a wide range of diseases, including cancer, autoimmune disorders, and infectious diseases. These biologics are designed to recognize and bind specific antigens, providing high specificity and minimal off-target effects, which makes them invaluable in both treating and managing complex conditions. As the use of mAb therapies continues to expand, understanding their pharmacokinetics and optimizing their therapeutic efficacy are critical for maximizing patient outcomes.
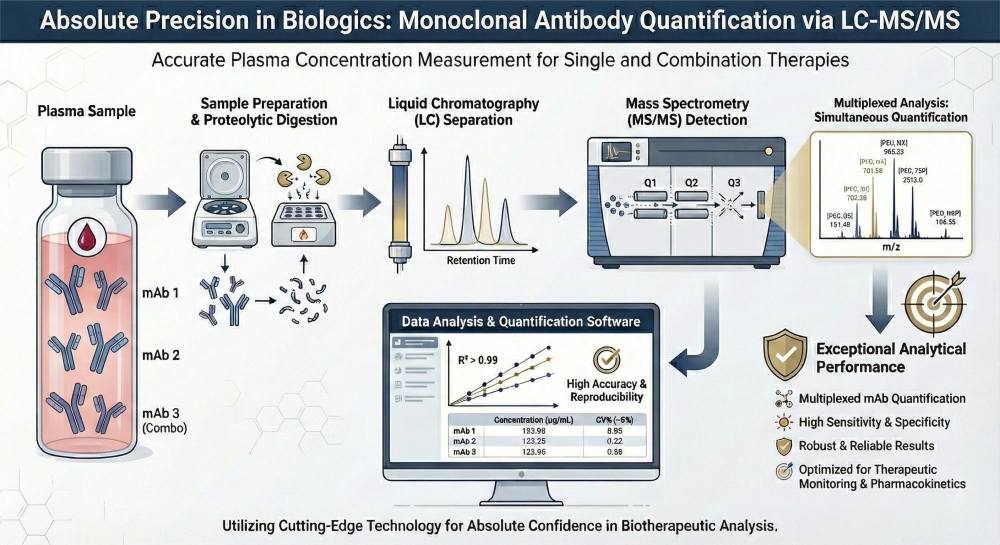
The quantification of monoclonal antibodies (mAbs) at specific time points using LC-MS/MS (liquid chromatography-tandem mass spectrometry) is a highly advanced technique that offers precise, accurate, and sensitive measurement of mAb concentrations in biological samples. This method relies on the separation of target molecules via liquid chromatography followed by detection and quantification through mass spectrometry, enabling detailed pharmacokinetic profiling. By measuring mAb levels at multiple time points, this approach allows for a comprehensive understanding of the drug's behavior in the body, including its half-life, peak concentration, and clearance rate.
The significance of this technology lies in its ability to provide reliable data that supports the optimization of dosing regimens, monitoring of therapeutic effectiveness, and assessment of immune responses, such as the presence of anti-drug antibodies (ADAs). Additionally, LC-MS/MS quantification plays a crucial role in ensuring regulatory compliance, safety monitoring, and personalized treatment strategies. This method is essential in clinical trials, drug development, and therapeutic monitoring, where precise and reproducible data are required for maximizing therapeutic efficacy and minimizing adverse effects.
Why Switch to MS-Based Quantification?
For decades, Ligand Binding Assays (LBAs), such as ELISA, were the standard. However, as biotherapeutics become more complex, LBAs face critical challenges: cross-reactivity, interference from Anti-Drug Antibodies (ADAs), and the inability to distinguish between structurally similar drugs.
Our LC-MS/MS service solves these challenges:
- Multiplexing Capabilities: In modern oncology and immunology, combination therapies are common. ELISA requires separate assays for each drug, consuming double the sample volume. Our MS technology performs multiplex analysis, simultaneously quantifying multiple mAbs in a single run with zero cross-talk.
- Superior Specificity: By targeting peptide sequences unique to the therapeutic antibody (and absent in the patient's endogenous IgG), we eliminate the "background noise" that plagues varying patient populations.
- Insensitivity to ADA Interference: Auto-antibodies produced by the patient can mask the epitopes required for ELISA binding, leading to false negatives. Mass spectrometry digests the sample, breaking down these complexes and allowing for the measurement of the total drug concentration.
Precise Quantification Of Monoclonal Antibodies at Creative Proteomics
This Monoclonal Antibody (mAb) Absolute Quantification Testing Service is designed for bioanalytical studies requiring accurate and reproducible measurement of therapeutic antibodies in complex biological matrices such as plasma or serum. Built on validated LC-MS/MS workflows, the service enables precise absolute quantification without the need for extensive in-house method development, making it suitable for both preclinical and clinical research applications.
The service employs stable isotope–labeled internal standards, including signature peptides or universal isotope-labeled antibody standards, to ensure high stoichiometric accuracy and robust inter-sample comparability. Optimized sample preparation workflows—covering protein denaturation, reduction/alkylation, enzymatic digestion, and peptide cleanup—are applied to maximize digestion efficiency and peptide recovery from complex matrices. Quantification is performed using verified MRM-based methods on triple-quadrupole LC-MS/MS platforms, ensuring high sensitivity, specificity, and reproducibility.
This testing service is widely used for pharmacokinetic (PK) and toxicokinetic (TK) studies, biosimilar development, bioequivalence assessment, and therapeutic antibody monitoring. Its popularity stems from the combination of analytical rigor, standardized workflows, and rapid turnaround, allowing researchers to obtain high-quality quantitative mAb data while focusing on study design and biological interpretation rather than assay optimization.
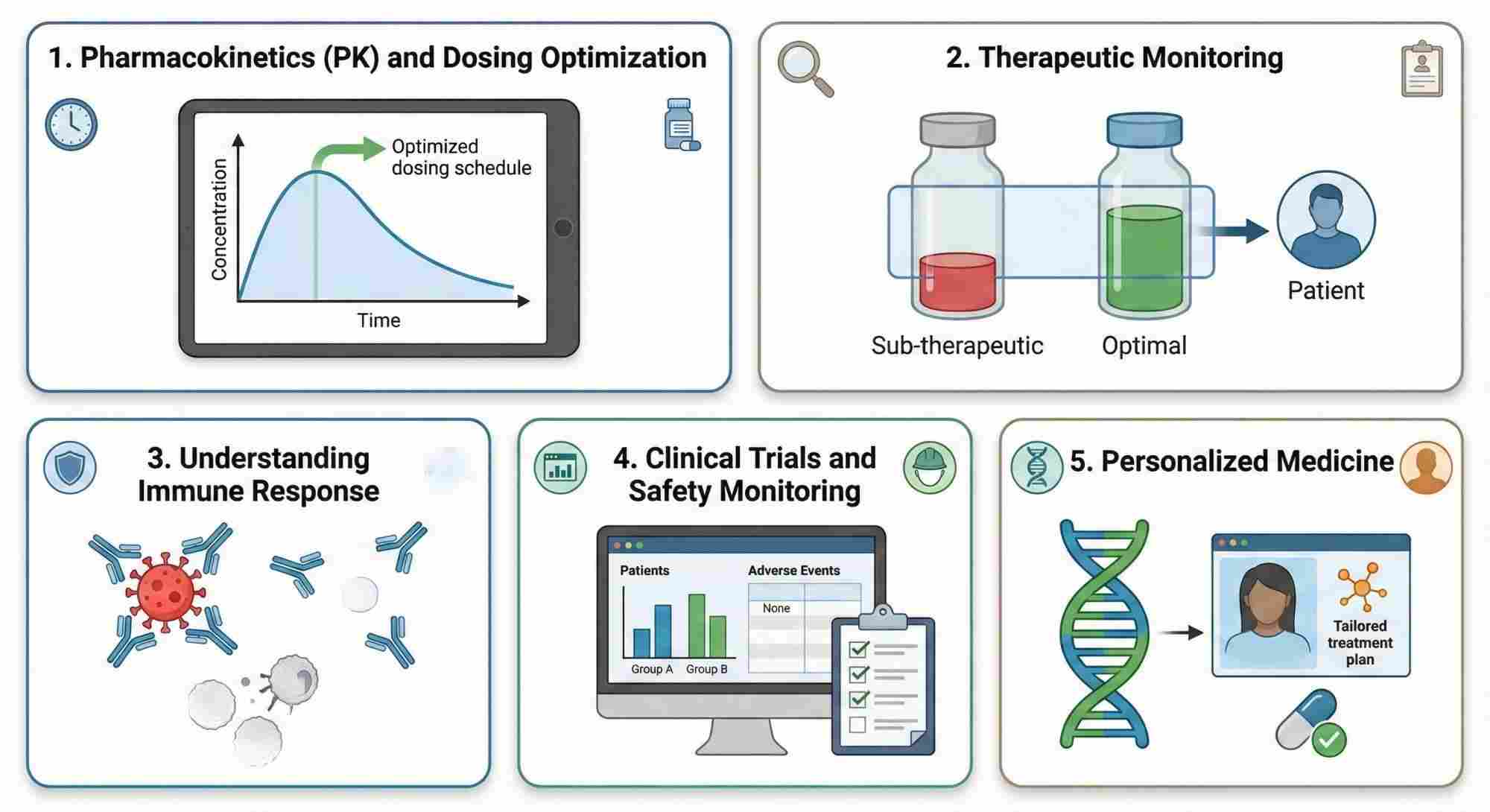
Applications of Monoclonal Antibody Quantification
- Pharmacokinetics (PK) and Dosing Optimization:
Quantifying mAbs at various time points helps to build the pharmacokinetic profile of the drug. This profile includes parameters like half-life, peak concentration (Cmax), and time to reach peak concentration (Tmax), which are crucial for determining the optimal dosing regimen.
By understanding how the mAb behaves in the body over time, scientists and clinicians can optimize the dose and frequency of administration for maximum therapeutic efficacy while minimizing side effects.
- Therapeutic Monitoring:
Tracking the concentration of mAbs over time allows clinicians to monitor the drug's therapeutic window. If mAb concentrations fall too low, efficacy may be compromised; if concentrations are too high, the risk of adverse effects increases.
In certain conditions, such as cancer treatment, high levels of therapeutic antibodies may be needed initially, while lower levels might suffice for maintenance therapy.
- Understanding Immune Response:
Measuring mAb levels at different time points can provide insights into how the immune system interacts with the drug. For example, the appearance of anti-drug antibodies (ADAs) might alter the pharmacokinetics of mAbs, leading to accelerated clearance and reduced efficacy.
Precise quantification also helps to track how the mAb concentration correlates with the progression or regression of a disease, which can guide therapeutic adjustments.
- Clinical Trials and Safety Monitoring:
In clinical trials, precise quantification is used to assess the consistency and reliability of the mAb's effect in patients over time. Tracking how patients' mAb levels evolve can identify any potential issues with drug administration, formulation stability, or metabolism.
It also allows for early detection of any toxicity, providing an additional layer of safety monitoring.
- Personalized Medicine:
Precise quantification at various time points can be used to tailor mAb therapy to individual patients. By understanding how a specific patient metabolizes and responds to a mAb, clinicians can adjust the treatment plan for better outcomes.
FAQs
-
What types of samples can be analyzed with this service?
The service quantifies monoclonal antibodies in plasma samples, commonly used in therapeutic and clinical research applications. It is suitable for both preclinical and clinical studies, including drug development, patient monitoring, and regulatory assessments.
-
Can the service quantify multiple monoclonal antibodies in a single sample?
Yes, our service supports multiplexed analysis, allowing for the simultaneous quantification of multiple monoclonal antibodies in a single biological sample. This capability enhances throughput and provides a comprehensive overview of mAb concentrations, essential for combination therapies.
-
Can your method distinguish between the therapeutic mAb and endogenous immunoglobulins?
Yes. We select "signature peptides" that are unique to the variable region (CDR) of your specific monoclonal antibody. These sequences do not exist in endogenous human (or animal) immunoglobulins, ensuring that the signal we detect comes only from your drug, eliminating the "background noise" often seen in non-specific immunoassays.
-
Can you quantify "Total" vs. "Free" mAb concentrations?
Yes. Total mAb: We generally digest the sample directly or use a generic capture method, which releases any bound drug, allowing us to measure the total amount of mAb present.
Free mAb: By using specific sample preparation techniques (like non-denaturing immunocapture) prior to MS analysis, we can isolate and quantify only the unbound fraction of the drug.
-
What is the minimum sample volume required?
One advantage of our modern LC-MS/MS workflows is low volume requirements. We typically require only 20–50 µL of serum or plasma per replicate, which is ideal for preclinical studies involving small animals (e.g., mice) or scarce clinical samples.
Sample Submission Guidelines
To ensure the highest quality data for your pharmacokinetic studies:
- Matrix: Plasma (EDTA or Heparin) and Serum are both acceptable.
- Sample Volume: Low volume requirements (typically 20–50 µL), ideal for microsampling and rodent studies.
- Storage: Samples should be separated from whole blood within 1 hour of collection and stored at -80°C.
- Labeling: Please label samples clearly with Time Point, Subject ID, and Group.
- Shipping: Ship on sufficient Dry Ice to prevent thawing during transit.

