Integrative Analysis Services of PTMs and Metabolomics
What is Integrative Analysis of PTMs and Metabolomics?
Post-translational modifications (PTMs) are chemical changes to proteins that alter their activity, stability, or localisation. These modifications include phosphorylation, acetylation, ubiquitination, glycosylation, and others. They act as molecular switches that control how proteins function in cells. Metabolites, the small molecules produced by cellular metabolism, reflect the functional state of biochemical pathways.
Proteins and metabolites are tightly interconnected. For example, the activity of a metabolic enzyme can be switched on or off by phosphorylation, which directly affects the production of metabolites. Conversely, specific metabolites can influence PTMs as substrates, cofactors, or regulators for modifying enzymes. Studying these layers separately often provides incomplete information.
Why is Integrative Analysis Necessary?
Studying PTMs or metabolites in isolation provides only a partial view of cellular regulation. PTMs act as molecular switches, controlling proteins' activity, stability, and localisation, including key enzymes in metabolic pathways. Metabolites, on the other hand, reflect the functional output of these enzymes and provide a snapshot of the cell's biochemical state.
Integrating PTMs with metabolite analysis is essential because it links cause and effect at the molecular level. For instance, a protein may be phosphorylated, but the biological consequence remains unclear without knowing how this modification changes metabolite levels. Similarly, observing a shift in metabolite concentration does not reveal which protein modifications drive the change. This integrative approach uncovers the direct connections between protein regulation and metabolism, enabling researchers to:
- Identify metabolic drivers of PTM-dependent signalling.
- Clarify how metabolic reprogramming impacts protein networks.
- Discover biomarkers that combine both molecular layers for higher specificity.
- Provide mechanistic insights into disease processes where protein modifications and metabolism are jointly altered.
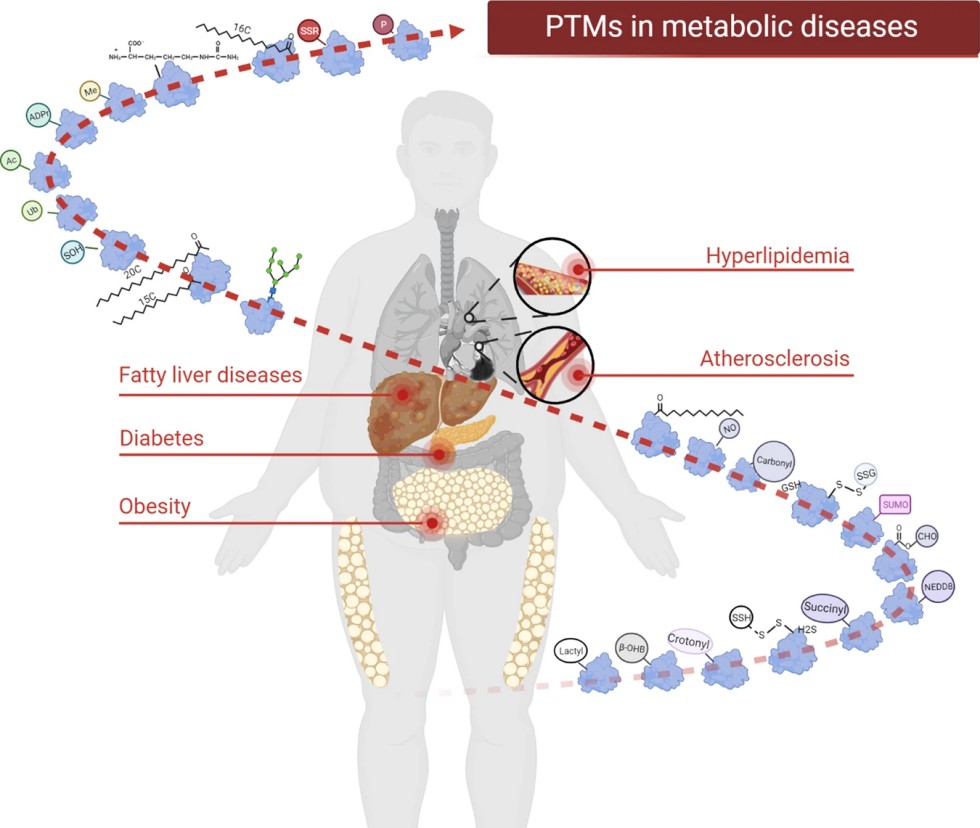
Figure 1. PTMs in metabolic diseases (Wu X, et al., 2023).
Common PTMs in metabolic regulation
| PTM Type | Target Proteins / Enzymes | Biological Role in Metabolic Regulation | Mechanistic Notes |
| Phosphorylation | Kinases, metabolic enzymes, transcription factors. | Rapid modulation of enzyme activity, signall transduction, nutrient sensing. | Reversible addition of phosphate groups to Ser, Thr, Tyr residues; triggers conformational changes, activation/inhibition. |
| Acetylation | Histones, metabolic enzymes (e.g., glycolytic and TCA cycle enzymes). | Links energy status to transcriptional and metabolic control; modulates enzyme activity and protein-protein interactions. | Acetylation via acetyl-CoA; can activate or inhibit enzymes depending on site and context. |
| Ubiquitination | Metabolic enzymes, transcription factors, signalling proteins. | Controls protein stability, turnover, and degradation; regulates flux through metabolic pathways. | Add ubiquitin to lysine residues; can target protein for proteasomal or lysosomal degradation. |
| Glycosylation | Secreted proteins, membrane transporters, enzymes. | Modulates enzyme activity, stability, and localisation; regulates secretion and signalling. | Addition of glycans to Asn, Ser, or Thr residues; affects folding and trafficking. |
| Methylation | Histones, transcription factors, metabolic enzymes. | Epigenetic and transcriptional regulation of metabolic genes; modulates protein activity. | Lysine or arginine methylation; can activate or repress transcription, alter enzyme function. |
| Disulfide bond formation | Enzymes, redox-sensitive proteins. | Stabilizes protein structure; regulates redox-sensitive metabolic enzymes. | Formation of covalent S–S bonds between cysteine residues; can switch enzyme activity in response to oxidative stress. |
Advantages of Our Integrative Analysis Services of PTMs and Metabolomics
- Comprehensive molecular insight: Linking PTM landscapes with metabolite fluxes for deeper biological interpretation.
- High-resolution analysis: Advanced mass spectrometry platforms for precisely identifying PTMs and quantifying metabolites.
- Customizable panels: Tailored analysis of phosphorylation, acetylation, ubiquitination, lipidation, and other PTMs alongside central metabolic pathways.
- Advanced bioinformatics: Multi-layer data integration pipelines to reveal regulatory connections and generate actionable hypotheses.
- Scalability: Suitable for small exploratory projects and extensive cohort studies across multiple conditions.
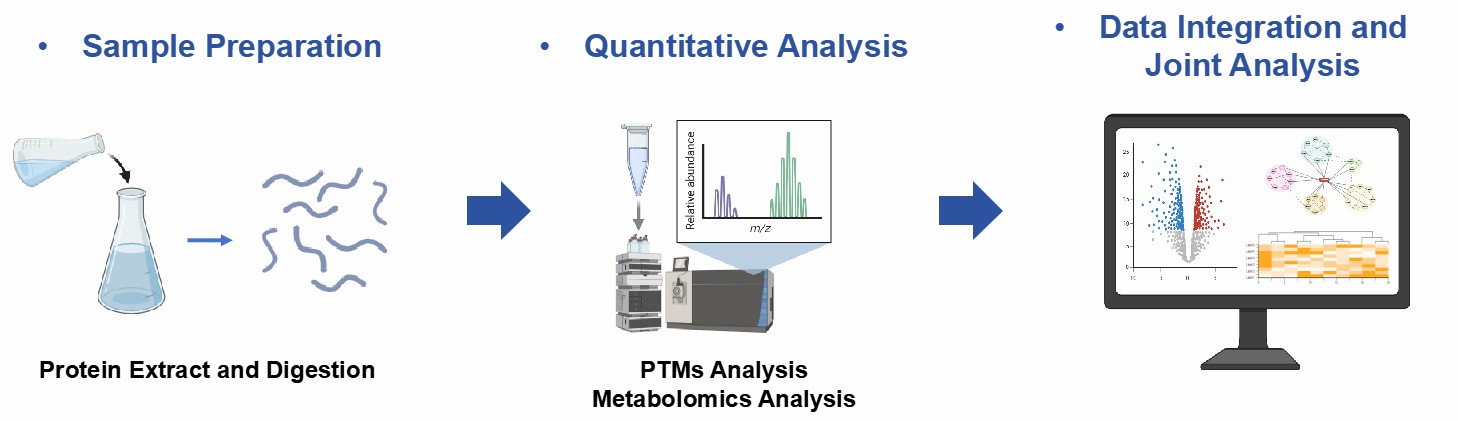
Workflow for PTMs and Metabolomics Integration Analysis
Deliverables and Reporting Standards
Raw Data and Instrument Files
- Proteomics: All raw LC-MS/MS data files should be included, covering both global proteome and PTM-enriched datasets.
- Metabolomics: Raw chromatograms, peak integrations, and instrument-specific output files from targeted assays (SRM/PRM or high-resolution MS) should be provided.
Processed Quantitative Data
- Proteome and PTM datasets: Include normalized protein abundances, PTM site-specific quantitation, fold changes, and associated statistical metrics.
- Metabolite quantification: Provide absolute or relative concentrations, calibration curves, internal standard corrections, and quality control (QC) metrics.
- Matched datasets: Clearly indicate which proteome/PTM samples correspond to each metabolomics sample to facilitate integrative analysis.
Visualizations for Interpretation
- Heatmaps: Differential PTM sites and metabolite abundances, showing clustering patterns and trends across conditions.
- Volcano plots: Highlighting statistically significant changes in proteins, PTMs, and metabolites.
- KEGG/Reactome pathway maps: Annotated with fold changes and significance to visualize integrated molecular pathways.
- Correlation networks: PTM–metabolite association networks displaying magnitude, directionality, and statistical confidence.
- Site-specific protein maps: For proteins with multiple PTMs, visual representation of modification sites and their quantitative changes.
Applications of PTM and Metabolomics Integration Analysis
- Drug Discovery and Pharmacology: PTM–metabolomics integration allows researchers to track how drugs affect protein function and metabolic pathways.
- Biomarker Discovery and Disease Monitoring: Combined PTM and metabolite profiling enhances the identification of molecular signatures associated with disease states. Researchers can pinpoint modified proteins and correlated metabolites that indicate early-stage disease or progression.
- Cancer and Metabolic Disorders: Cancer cells and metabolic diseases often reprogram their energy usage and biosynthetic pathways. Integrated analysis reveals how PTMs regulate enzymes controlling these pathways, uncovering mechanisms driving abnormal cell growth and energy imbalance.
- Systems Biology and Mechanistic Insights: By mapping PTM changes alongside metabolite profiles, researchers can reconstruct regulatory networks that connect signalling pathways to metabolic outputs.
- Agricultural and Microbial Research: In plant and microbial studies, integrated analysis helps reveal how protein modifications respond to environmental stressors and affect growth or yield.
Simple Requirements
| Sample Type | Minimum Quantity Required | Storage Conditions | Special Considerations |
| Cell Pellets | ≥ 5 × 10⁶ cells | Snap-freeze in liquid nitrogen; store at –80°C. | Avoid repeated freeze-thaw cycles; wash with PBS before storage. |
| Tissue Samples | ≥ 50 mg | Snap-freeze immediately; store at –80°C. | Homogenize quickly to prevent PTM degradation. |
| Serum / Plasma | ≥ 200 µL | Store at –80°C; avoid hemolysis. | Use EDTA or heparin tubes; minimize exposure to room temperature. |
| Urine Samples | ≥ 500 µL | Store at –80°C after centrifugation. | Collect midstream; avoid preservatives unless specified. |
| Protein Extracts | ≥ 100 µg total protein | Store at –80°C in aliquots. | Quantify accurately; provide buffer composition for reproducibility. |
| Metabolite Extracts | ≥ 200 µL | Store at –80°C under inert gas if possible. | Avoid contamination; record solvent details. |
| labellled Samples (optional) | ≥ 100 µg | Store at –80°C. | Provide labelling strategy and isotopic information for integration. |
Why Choose Creative Proteomics
- Integrated Multi-Omics Expertise: Two decades of experience in PTM characterization and targeted metabolomics.
- Advanced Analytical Platforms: High-resolution LC-MS/MS combined with state-of-the-art bioinformatics pipelines.
- Data Interpretation Support: Comprehensive reports with pathway mapping, statistical analysis, and biological insights.
- Global Research Enablement: Trusted by pharmaceutical companies, biotech firms, and academic institutions worldwide.
FAQ
-
Q1: What sample types are compatible with integrative PTM-metabolomics?
A1: Cells, tissues, plasma, serum, cerebrospinal fluid, urine, and extracts from plants or microbes are compatible. Choose matrixes based on the biological question. Preserve samples at -80°C.
-
Q2: How to choose targeted metabolomics or non-targeted?
A2: Targeted assays are ideal for testing specific hypotheses and achieving precise, absolute quantification of molecules. They are particularly advantageous when high sensitivity, reproducibility, and measurement accuracy are critical. Targeted panels allow focused analysis of selected metabolites or proteins, minimizing background noise and enabling reliable comparisons across samples or experimental conditions.
-
Q3: How are low-abundance PTMs or metabolites handled?
A3: Enrichment strategies, highly sensitive mass spectrometry, and targeted metabolomics (MRM/PRM) are essential. Combining fractionation with high-resolution LC-MS/MS maximizes detection coverage.
-
Q4: Can PTMs-metabolomics be applied to microbiome studies?
A4: Yes. Host-microbe interactions can be dissected by integrating microbial PTMs with metabolite fluxes, revealing metabolic crosstalk and signalling dynamics.
-
Q5: What validation strategies are advised?
A5: Validation may involve targeted MS (SRM/PRM), isotope labelling, enzymatic assays, or genetic perturbation (knockdown/overexpression of the modified protein).
Demo
Demo: Effects of moderate intensity exercise on liver metabolism in mice based on multi-omics analysis
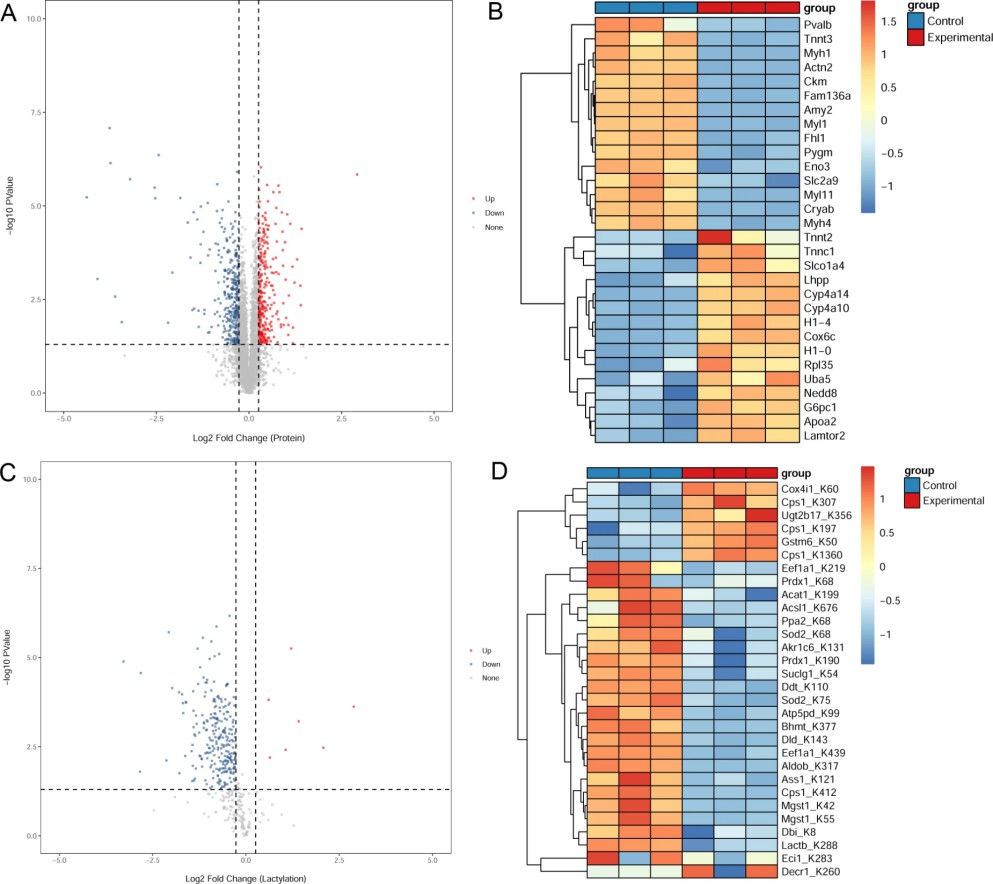
Figure 2. Proteomics and lactylation modification analyses (Wang F, et al., 2024).
-
Case Study
Case: Integrated phosphoproteomic and metabolomic profiling reveals perturbed pathways in the hippocampus of gut microbiota dysbiosis mice
Abstract
The study explores how imbalances in gut microbes affect brain function and behaviour through the microbiota-gut-brain axis. While previous research links gut microbiome changes to psychiatric disorders, the mechanisms remain unclear. To investigate this, the authors combine hippocampal phosphoproteomics with metabolomics from serum and hippocampal tissues to uncover molecular pathways connecting gut health to depression-like behaviours.
Methods
- Animal models: Germ-free (GF) versus specific pathogen-free (SPF) mice; fecal-microbiota-transplant (FMT) recipients colonized with "depression microbiota" or "healthy microbiota."
- Tissue/sample collection: Hippocampus tissue for phosphoproteomics; serum and hippocampus for metabolomics.
- Proteomic workflow: Mass-spectrometry-based phosphoproteomic profiling to identify and quantify phosphorylation sites.
- Metabolomic workflow: Unbiased metabolomics of serum and hippocampus to profile lipid and amino-acid metabolites.
- Data integration: For validation, joint pathway and enrichment analyses to link differential phosphosites with metabolite changes; cross-comparison to other phosphoproteomic datasets (stress models and human MDD postmortem tissue).
Results
- Phosphorylation changes: Significant dysregulation of phosphorylation was observed. The authors report hundreds of differential phosphosites.
- Metabolic disturbances: The integrated analysis showed consistent lipid and amino acid metabolism disruptions in both serum and hippocampus, indicating changes in metabolite profiles and lipid composition that may affect cell membranes and signalling.
- signalling pathways: CAMKII–CREB signalling emerged as a commonly perturbed neuronal pathway responding to metabolic and phosphorylation changes. Glutamatergic neurotransmission components showed altered phosphorylation.
- Novel findings: Dysbiosis affected spliceosome activity, a previously overlooked neuronal function. NCBP1 phosphorylation was identified as a potential pathogenic target. Cross-species analysis revealed shared phosphoproteins between animal models and human MDD tissues.
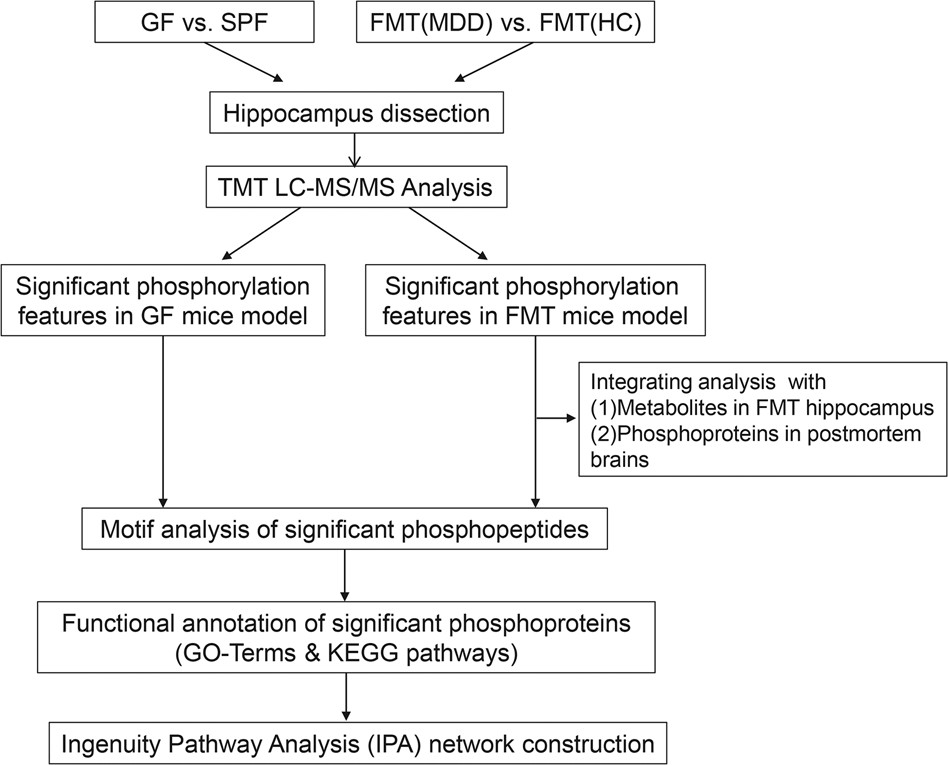
Figure 3. Stepwise experimental flowchart depicting the analysis of this study.
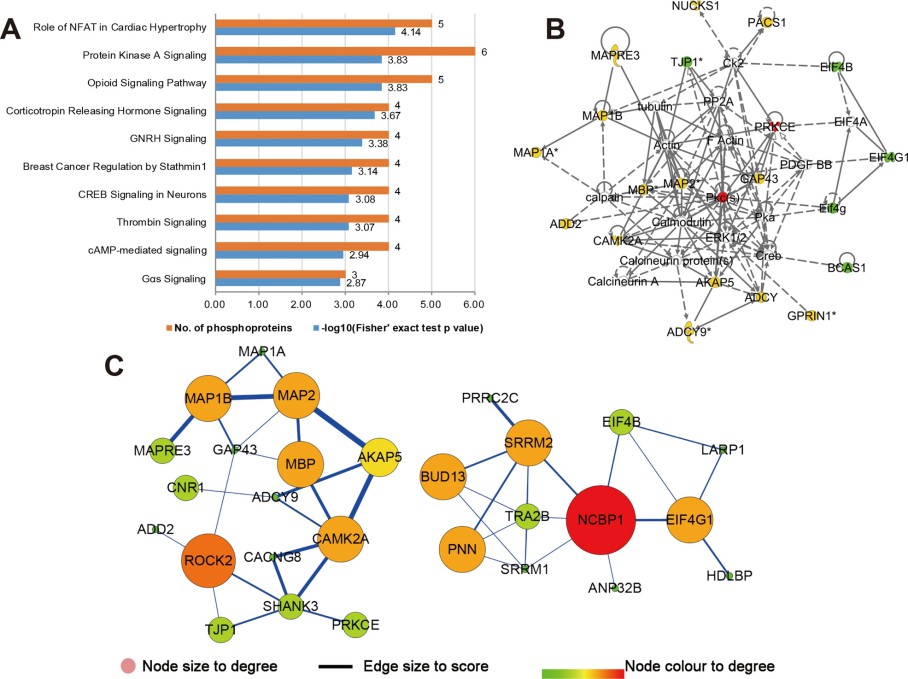
Figure 4. Bioinformatics analysis for the microbial-specific phosphoproteins.
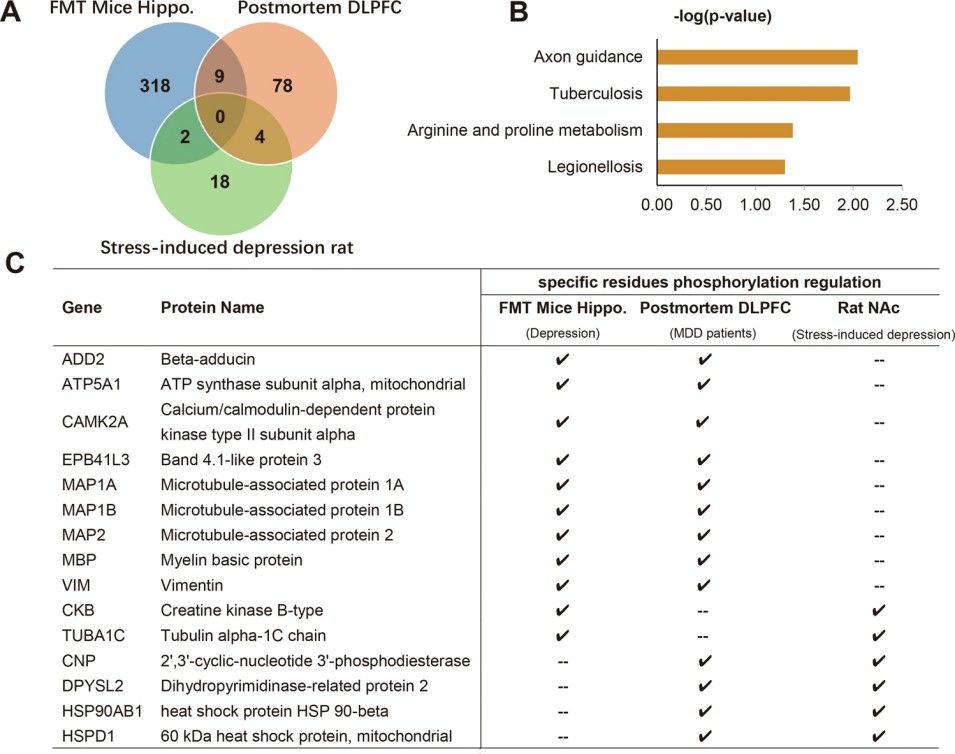
Figure 4. Integrated phosphoproteomic analysis of depressive rodents and patients with major depression disorders.
Conclusion
The authors conclude that an imbalance in gut microbiota can trigger depression-like behaviours by simultaneously altering protein phosphorylation and metabolism. Their integrated phosphoproteomic and metabolomic analysis revealed connections between lipid and amino acid metabolism changes and neuronal signalling, particularly the CAMKII–CREB and glutamate pathways. The study highlights potential molecular targets, such as NCBP1 phosphorylation, for further investigation. Limitations include the exclusive use of male mice, focus on the hippocampus, and the need for additional validation across broader samples.
Related Services
References
- Wu X, et al. Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. signall transduction and targeted therapy, 2023, 8(1): 220.
- Zhang B, Schroeder F C. Mechanisms of metabolism-coupled protein modifications. Nature Chemical Biology, 2025: 1-12.
- Simithy J, Sidoli S, Garcia B A. Integrating proteomics and targeted metabolomics to understand global changes in histone modifications. Proteomics, 2018, 18(18): 1700309.

