Electrochemiluminescence (ECL) Assay Service
What is Electrochemiluminescence (ECL)?
Electrochemiluminescence (ECL) is a luminescence phenomenon in which light is generated through an electrochemical reaction. In ECL-based immunoassays, chemical labels attached to detection antibodies are activated by a controlled electrical stimulus, causing them to emit light. Unlike traditional fluorescence or colorimetric detection, the excitation (electricity) is separate from the signal (light), which minimizes background noise and enables highly sensitive detection of biomolecules. ECL combines high sensitivity, broad dynamic range, and compatibility with multiplexed assays while requiring minimal sample volumes.
How Does ECL Work?
ECL is the detection principle used in plate-based ECL immunoassays to deliver high sensitivity and low background. Non-specific background signals are largely eliminated because only labels close to the electrode surface emit light upon electrical stimulation. The result is a clean, highly sensitive readout suitable for quantifying proteins at very low concentrations. The process proceeds as follows:
- Capture on the Electrode Surface: Assay plates contain electrodes integrated into the bottom of each well. These electrode regions serve as the detection zones where capture reagents immobilized on the surface bind target molecules from the sample.
- Labelling with Electrochemiluminescent Tags: Detection antibodies are conjugated to electrochemiluminescent labels (ECL tags). These labels emit light only when provided with an appropriate electrical stimulus in a defined chemical environment.
- Electrical Stimulation: A controlled electrical pulse is applied to the electrode, activating the ECL tags. Because excitation (electricity) is decoupled from the optical signal (light), only labels near the electrode contribute to the measured signal.
- Light Emission and Detection: Activated ECL tags emit a brief luminescent signal. The instrument records light intensity, which is proportional to the analyte concentration in the sample.
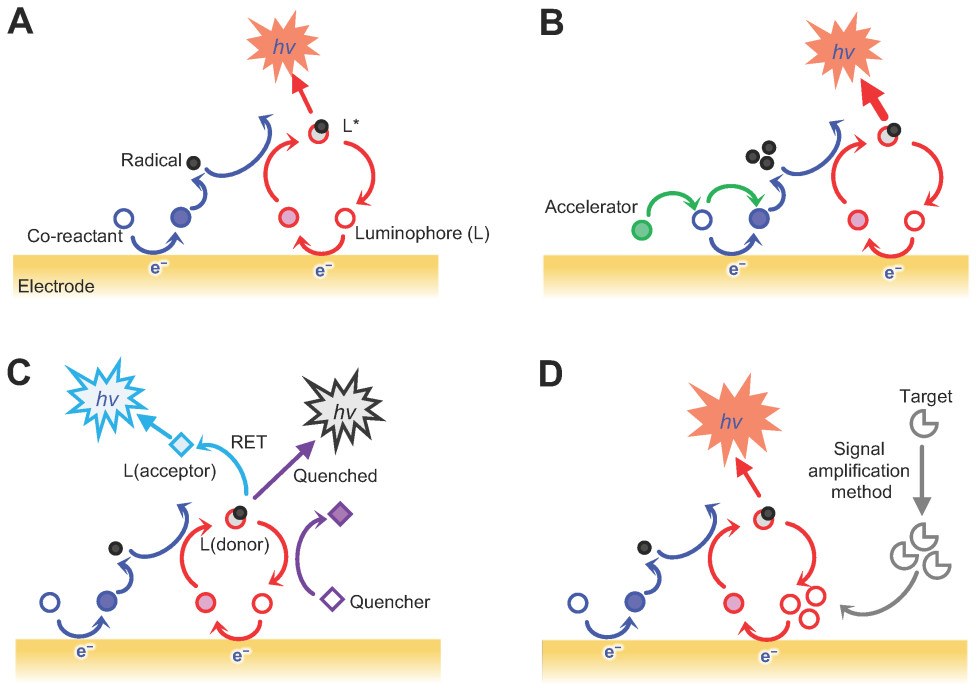
Figure 1. Schematic illustration of EC-sensing systems classified according to the reactions that drive the ECL signal emission (Yoo S M, et al., 2022).
Advantages of ECL Assays
- High sensitivity: Plate-based ECL immunoassays detect very low protein concentrations that conventional colorimetric ELISA may miss.
- Broad dynamic range: A single ECL assay can quantify high- and low-abundance analytes within the same sample.
- Low background noise: Only labels adjacent to the electrode are detected, minimizing stray light and non-specific signal.
- Multiplexing capability: Multiple biomarkers can be measured in defined detection sites within a single well, conserving sample volume—critical for limited clinical samples.
- Small sample volumes: ECL multiplex assays commonly require only microliters of sample.
- Flexible and adaptable: Laboratories may use validated panels, custom multiplex designs, or bespoke single-analyte assays according to project needs.
- High throughput and automation-friendly: Compatible with standard 96- and 384-well formats for efficient, automated workflows..
Differences between ECL, ELISA and Olink
| Feature / Parameter | Plate-based ECL platform | ELISA | Olink Proteomics |
| Detection Principle | Electrochemiluminescence; ECL labels on detection antibodies produce light upon electrode stimulation | Colorimetric (HRP/TMB), fluorescent, or luminescent enzymatic reactions | Proximity Extension Assay (PEA); dual oligonucleotide-labelled antibodies generate qPCR signal upon target binding |
| Multiplexing Capacity | Multiple analytes per well (typical commercial implementations: up to ~8–10 spots per well) | Single-analyte per well; multi-analyte requires multiple plates | Extremely high; 92–3072 proteins per panel |
| Sensitivity / LLOD | High; ultra-sensitive formats can reach low fg/mL range | Moderate; pg/mL range | Very high; low fg/mL range depending on panel |
| Sample Volume Required | Low; 10–25 μL per well for multiplex assays | Moderate; 50–100 μL per analyte | Very low; 1–5 μL per sample per panel |
| Throughput | High; 96-well plate, rapid read times, automated options | Low to moderate; multiple wash and incubation steps slow workflow | Moderate to high; 96- or 384-well plate, mostly automated |
| Assay Flexibility | High; validated kits, custom multiplex, or user-developed assays | Limited; minor customization only | Moderate; panel selection fixed, limited customizability |
| Best Use Cases | Biomarker panels, PK/PD studies, immunogenicity testing, cell/gene therapy studies | Single-protein quantification, low-throughput studies, validation of exploratory hits | Large-scale biomarker discovery, high-throughput profiling, cohort studies |
| Advantages | Multiplexing with low sample volumes, broad dynamic range, ultra-sensitivity, robust across matrices | Simplicity, established method, widely understood | Extremely high multiplexing, minimal sample consumption, consistent data across large panels |
| Limitations | Higher instrument cost, limited maximum multiplex (up to 10 per well), requires specialized plates | Single-analyte throughput, higher sample consumption, limited sensitivity | Panel dependence and proprietary workflows |
Workflow of Our ECL Assay Service
Our ECL assay service follows a streamlined workflow to deliver reproducible, publication-ready results:
- Sample preparation and quality assessment: Incoming samples (serum, plasma, cell culture supernatant, tissue lysate, CSF, urine, etc.) are QC-checked for integrity (hemolysis, lipemia, freeze-thaw), and suitability for analysis is recorded.
- Assay design and panel customization: We collaborate with clients to select validated panels or design custom singleplex/multiplex panels. Capture and detection reagents are optimized for specificity and sensitivity to the analytes of interest.
- Electrochemiluminescence detection: Samples are incubated in multi-spot plates with immobilized capture reagents; ECL-labeled detection antibodies are added and, following washing, application of an electrical stimulus triggers luminescence for signal acquisition.
- Data analysis and interpretation: Raw signals are processed into calibrated concentration values using standard curves. We provide statistical summaries, visualization, and biological interpretation focusing on project objectives.
- Reporting and consultation: Deliverables include raw data, processed tables, QC metrics, and an interpretive report with recommendations for next steps.
Applications of ECL in Biomedical Research
- Biomarker discovery and validation: Multiplex ECL panels facilitate profiling disease-associated protein signatures across cohorts with limited sample volumes.
- Pharmacodynamics and toxicology: Quantitative protein measures help monitor drug effects, target engagement, and safety biomarkers.
- Cytokine and immune profiling: Simultaneous measurement of cytokines and chemokines supports immunology and infection studies.
- Cell and gene therapy research: Sensitive quantification of therapeutic proteins, transgene products, and immune responses in complex matrices.
Simple Requirements for ECL Assay
| Sample Type | Serum, plasma, cell culture supernatants, whole blood, CSF, urine, tissue extracts |
| Sample Volume | Typically 10–50 µL per analyte (multiplexing reduces total volume needed) |
| Sample Quality | Fresh or properly stored (e.g., -80°C for plasma/serum), free of hemolysis or debris |
| Multiplexing Capability | Up to 10 analytes per well for standard panels; customizable for more analytes with special designs |
| Assay Format | Sandwich, direct, indirect, or bridging assay depending on project requirements |
Why Choose Creative Proteomics for ECL Assay
- Sample Preparation and Quality Control: Rigorous evaluation to ensure sample integrity.
- Customized Panel Design: Development of singleplex or multiplex panels tailored to research goals.
- ECL-Based Detection: ECL-based detection expertise with validated workflows for high sensitivity and dynamic range.
- Data Analysis and Interpretation: Quantitative analysis with robust bioinformatics support.
- Comprehensive Reporting: Delivery of publication-ready results and technical consultation.
FAQ
-
Q1: Can ECL multiplex assays reliably measure multiple analytes in one sample without cross‐reactivity or bias?
A1: Yes. Validated multiplex ECL assays (e.g., 8-plex for ETEC vaccine antigens, or SARS-CoV-2 antigen panels) have shown strong performance. These studies demonstrate good correlation with each analyte's ELISA, acceptable spike/recovery metrics, and reliable quantitation across sample types. Proper panel design and validation are essential to minimize cross‐reactivity.
-
Q2: How do you control for matrix effects and interference?
A2: We evaluate matrix effects via spike/recovery, dilution linearity, and parallelism testing across representative matrices; where required, we optimize diluents, blocking reagents, or alternate sample preparation to mitigate interference. Acceptance criteria are established during method qualification.
-
Q3: How is cross-reactivity evaluated in multiplex panels?
A3: Cross-reactivity is assessed by single-plex vs multiplex comparison, spiking individual analytes into pooled matrices, and testing potential interfering substances. Acceptable cross-reactivity thresholds and mitigation strategies are defined during panel design.
Demo
Demo: Sensitive electrochemiluminescence (ECL) immunoassays for detecting lipoarabinomannan (LAM) and ESAT-6 in urine and serum from tuberculosis patients
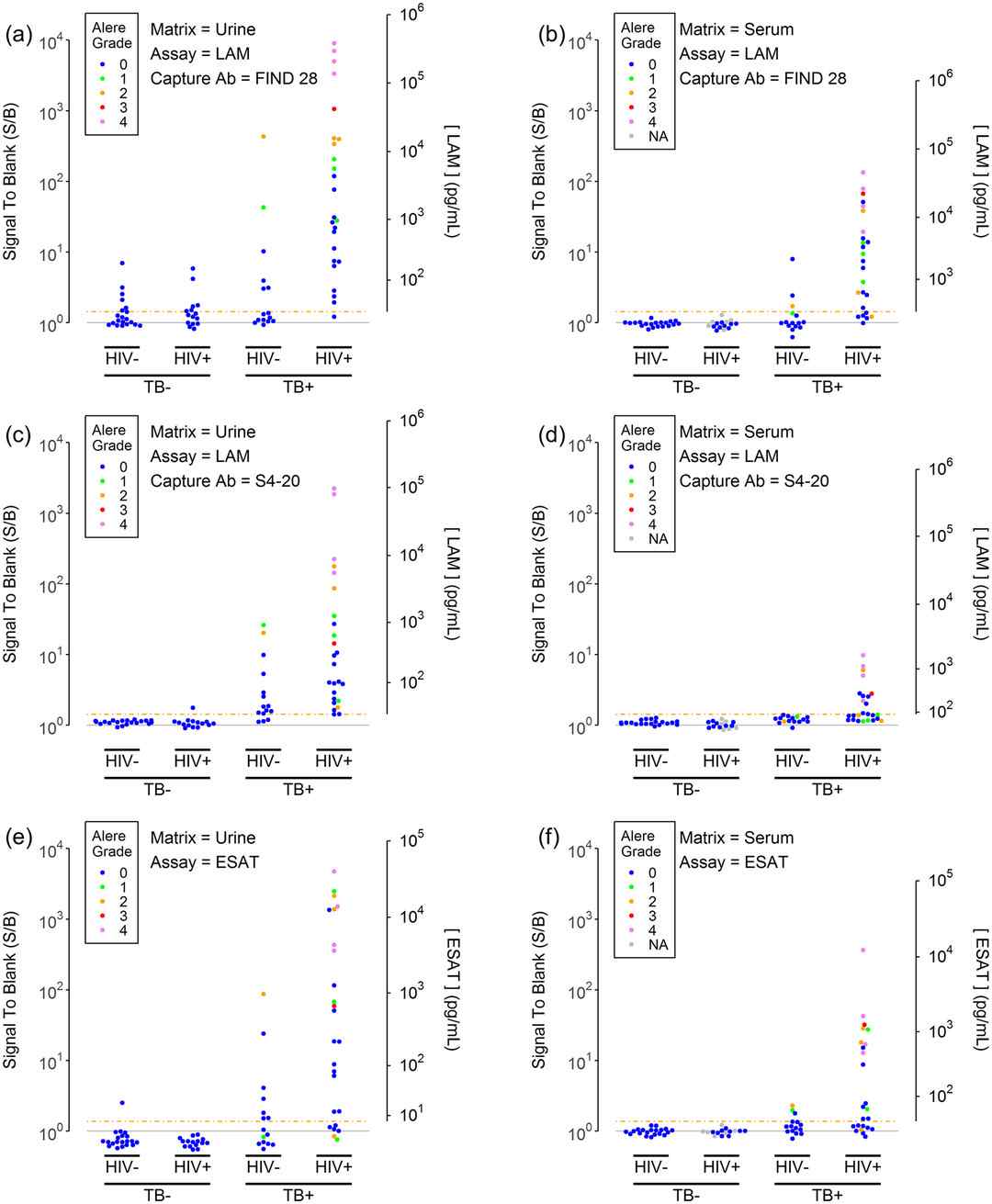
Figure 2. LAM and ESAT-6 concentrations in urine and serum samples (Broger T, et al., 2019).
-
Case Study
Case: An electrochemiluminescence based assay for quantitative detection of endogenous and exogenously applied MeCP2 protein variants.
Abstract:
Methyl-CpG-binding protein 2 (MeCP2) is a chromosomal protein crucial for gene regulation in the central nervous system. Both deficiency and overexpression of MeCP2 cause severe neurological disorders, including Rett syndrome (RTT) and MECP2 duplication syndrome. Current RTT treatments are limited, and protein replacement therapy using TAT-MeCP2 fusion proteins has emerged as a potential therapeutic approach. Accurate, sensitive quantification of MeCP2 is essential for developing such therapies. To express and purify recombinant TAT-MeCP2 fusion proteins and develop a sensitive, high-throughput electrochemiluminescence immunoassay (ECLIA) capable of measuring endogenous and exogenously applied MeCP2 protein levels in cells and brain tissue, supporting therapeutic and uptake studies.
Methods
- TAT-MeCP2 and TAT-MeCP2-eGFP fusion proteins were expressed in E. coli and purified using biotin-binding affinity purification resin.
- Human fibroblasts (healthy and MeCP2-deficient), HEK-293, NSC-34 cells, and mouse brain tissues were prepared for protein extraction.
- A 96-well ECLIA was established using specific capture and detection antibodies, with electrochemiluminescence for quantitative readout.
- In vitro BBB permeation studies were performed using cerebEND endothelial cells to evaluate TAT-MeCP2 transport.
Results
- Milligram quantities of stable, purified TAT-MeCP2 and TAT-MeCP2-eGFP were obtained.
- The ECLIA showed high sensitivity (LLOD 1.002 ng/mL), broad dynamic range (1–1800 ng/mL), low intra- and inter-assay variability, and superior performance compared to Western blot and commercial ELISA kits.
- Endogenous MeCP2 levels were accurately measured in human and mouse samples, including detecting differences between wild-type and heterozygous mice.
- TAT-MeCP2 uptake studies demonstrated time- and concentration-dependent nuclear accumulation in MeCP2-deficient fibroblasts.
- In vitro BBB studies confirmed the ability of TAT-MeCP2 and TAT-MeCP2-eGFP to cross endothelial cell layers.
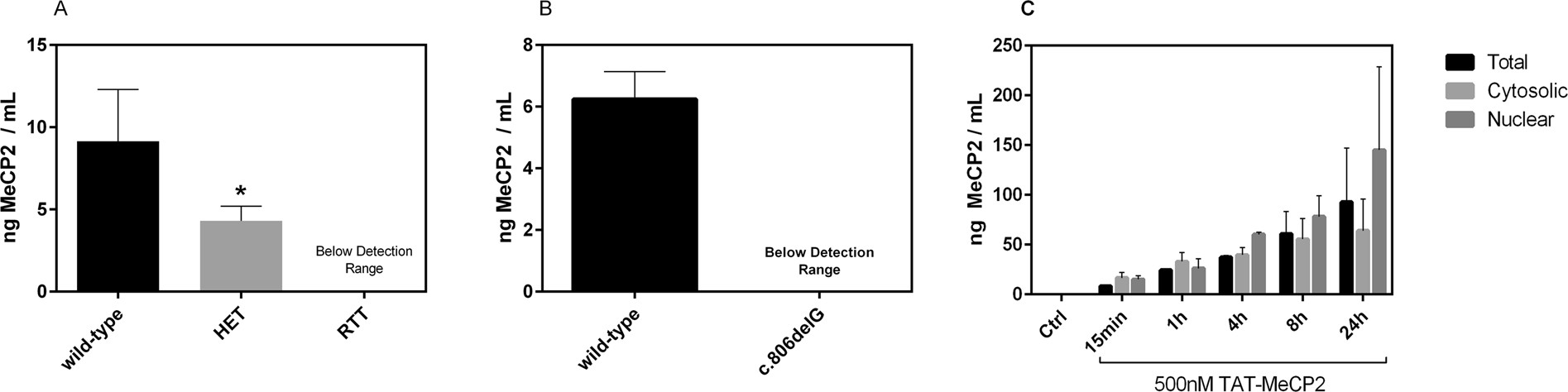
Figure 3. Determination of Mecp2 protein levels in mouse brains and human fibroblasts via MeCP2 ECLIA assay.
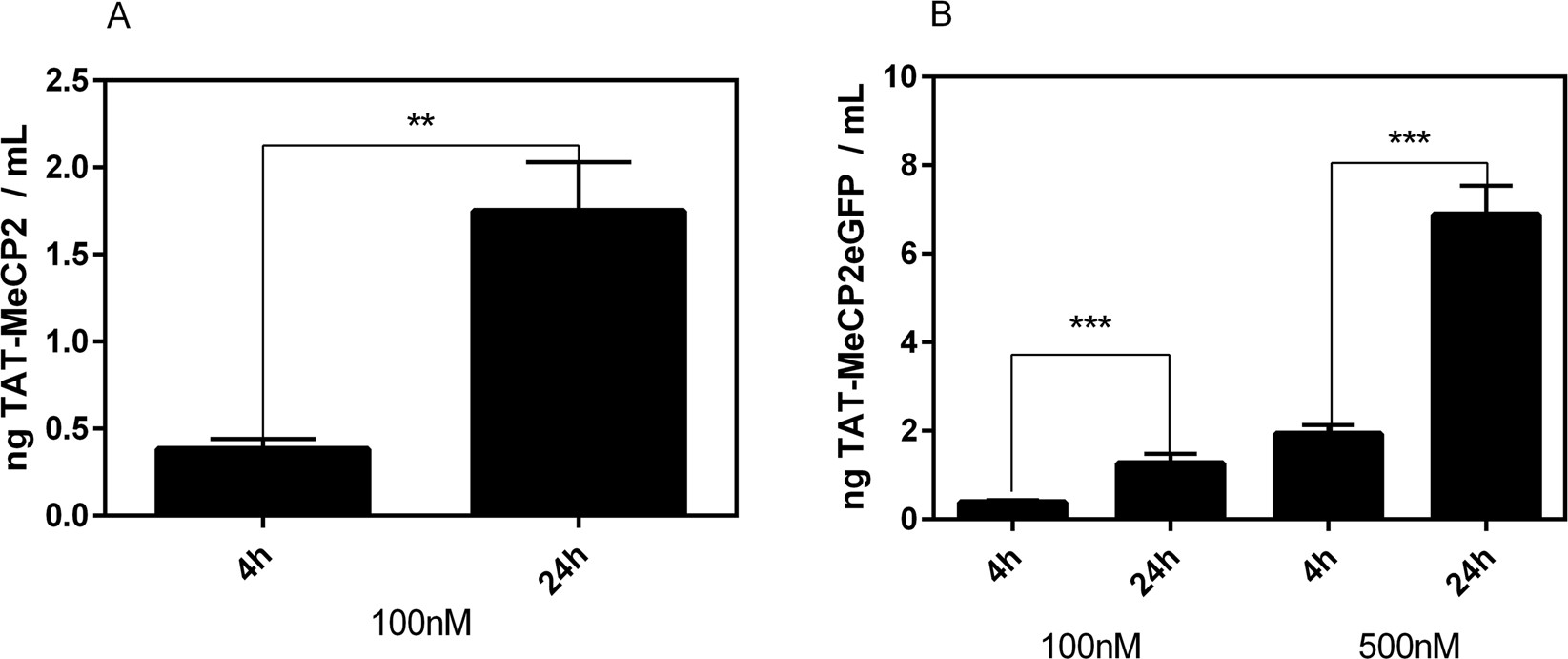
Figure 4. Transport of TAT-MeCP2 and TAT-MeCP2-eGFP across a blood-brain barrier in vitro model.
Conclusion
The study successfully produced recombinant TAT-MeCP2 fusion proteins and established a quantitative, sensitive ECLIA for measuring MeCP2 levels. This platform enables precise dosage assessment for protein replacement therapy and facilitates studies on MeCP2 dynamics, including BBB transport, providing a valuable tool for RTT therapeutic development.
Related Services
References
- Yoo S M, Jeon Y M, Heo S Y. Electrochemiluminescence systems for the detection of biomarkers: strategical and technological advances. Biosensors, 2022, 12(9): 738.
- Broger T, et al. Sensitive electrochemiluminescence (ECL) immunoassays for detecting lipoarabinomannan (LAM) and ESAT-6 in urine and serum from tuberculosis patients. PloS one, 2019, 14(4): e0215443.
- Steinkellner H, et al. An electrochemiluminescence based assay for quantitative detection of endogenous and exogenously applied MeCP2 protein variants. Scientific reports, 2019, 9(1): 7929.

