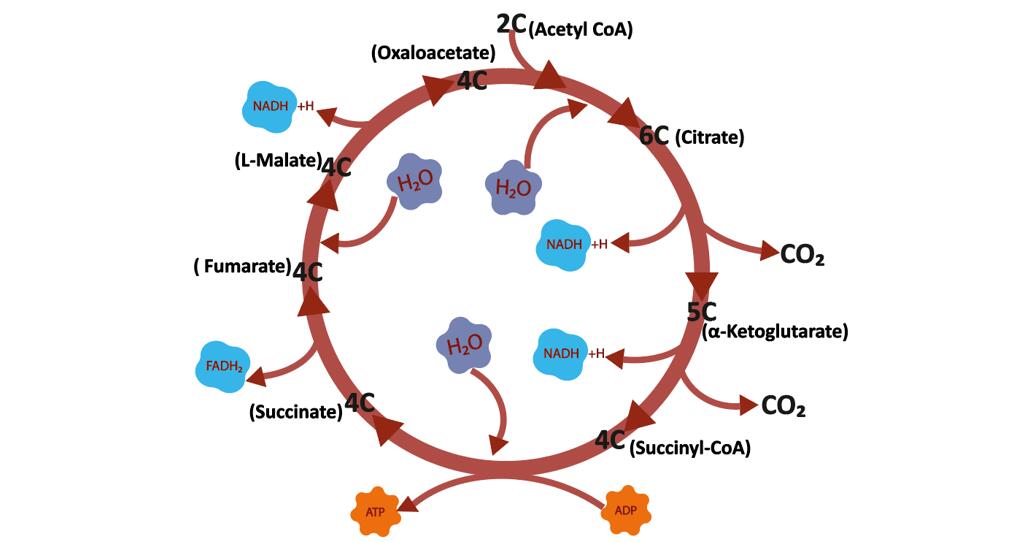
The tricarboxylic acid cycle (TCA Cycle) is a cyclic reaction system consisting of a series of enzymatic reactions, starting with the formation of citric acid from acetyl coenzyme A and oxaloacetate, followed by four dehydrogenations, a horizontal phosphorylation of the substrate, and finally the production of two molecules of carbon dioxide and the re-formation of oxaloacetate.
TCA Cycle
Significance of TCA Cycle
The TCA cycle is the main way in which the body obtains energy. 1 molecule of glucose produces only 2 molecules of ATP net through anaerobic digestion, while aerobic oxidation produces 38 molecules of ATP net, including 24 molecules of ATP from the tricarboxylic acid cycle. Under normal physiological conditions, many tissue cells obtain energy from aerobic oxidation of sugars. The aerobic oxidation of sugar is not only efficient in releasing energy, but also releases energy gradually and stores it in ATP molecules, thus making it highly energy efficient.
The TCA cycle is a common metabolic pathway for the complete oxidation of the three major organic substances: sugar, fat and protein. The starting material of the TCA cycle, acetyl-CoA, is not only a product of sugar oxidation and decomposition, but it can also come from the metabolism of glycerol and fatty acids from fats and some amino acids from proteins. Therefore, the tricarboxylic acid cycle is actually a common pathway for the oxidation and energy supply of the three major organic substances in the body, and it is estimated that 2/3 of the organic substances in the human body are broken down through the tricarboxylic acid cycle.
The TCA cycle is the liaison mechanism for the interchange of the three major organic compounds in the body. The metabolism of sugars and glycerol in the body can produce intermediates of the TCA cycle such as α-ketoglutarate and oxaloacetate. These intermediates can be transformed into certain amino acids. Some amino acids can be transformed into α-ketoglutaric acid and oxaloacetic acid through different pathways, and then into sugar or glycerol through the pathway of gluconeogenesis. Thus, the tricarboxylic acid cycle is not only the ultimate common pathway for the catabolism of the three major organic compounds, but also the liaison mechanism for their mutual transformation.
The tricarboxylic acid cycle is the ultimate metabolic pathway for the complete oxidative breakdown of the three major nutrients (carbohydrates, lipids and amino acids) and the central hub of carbohydrate, lipid and amino acid metabolism. Detection of metabolites associated with the tricarboxylic acid cycle helps us to assess the energy metabolic state of cells. So which methods can be used for TCA analysis?
How to Analyze TCA Cycles?
- LC-MS/MS analysis techniques
The LC-MS/MS platform enables the simultaneous relative and absolute quantification of multiple metabolites in biological systems. By studying the relative changes in metabolite concentrations, these results are widely used for biomarker discovery. The resulting data have been used extensively for biomarker discovery. By providing accurate molecular weights and retention times, LC-MS/MS technology has become a powerful analytical tool for the identification and quantification of small molecules. Creative Proteomics has established sensitive, reliable and accurate HPLC-MS/MS methods to quantify metabolites in the TCA cycle.
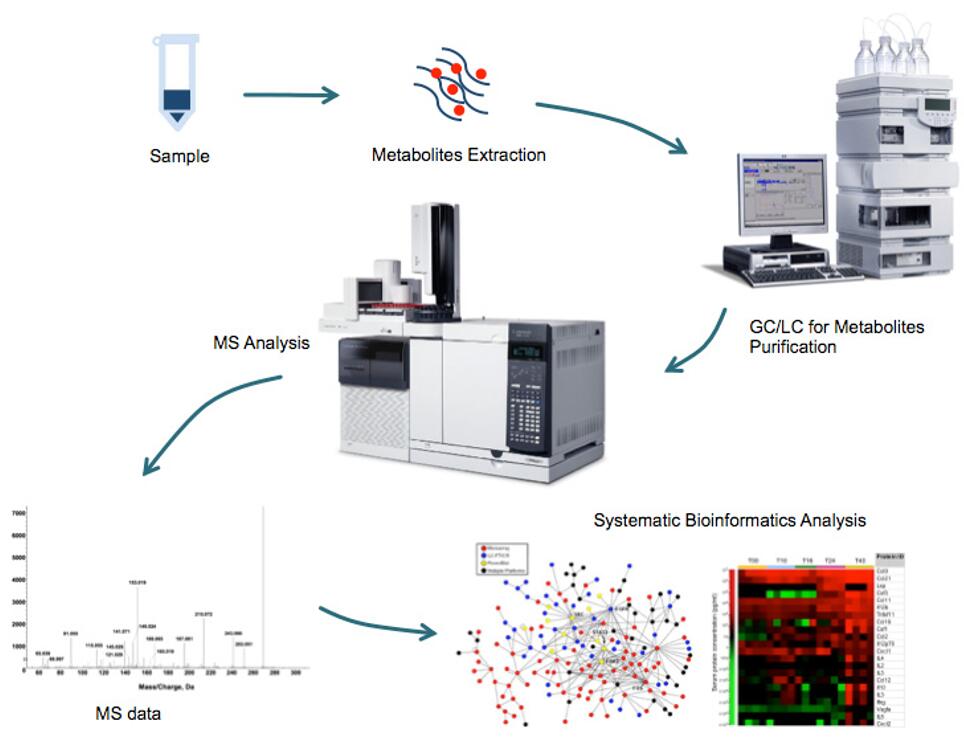
- Metabolic flow solutions
Metabolic flowomics (Fluxomics) is a new technology that evolves from traditional metabolomics (reflecting static metabolite content information). Metabolic flow analysis methods based on 13C or 15N labeling provide a comprehensive study of metabolite production and consumption flow rates (flux rates) by analyzing the stable isotope labeling patterns of downstream metabolites to provide a true picture of cellular metabolic activity during physiological processes.
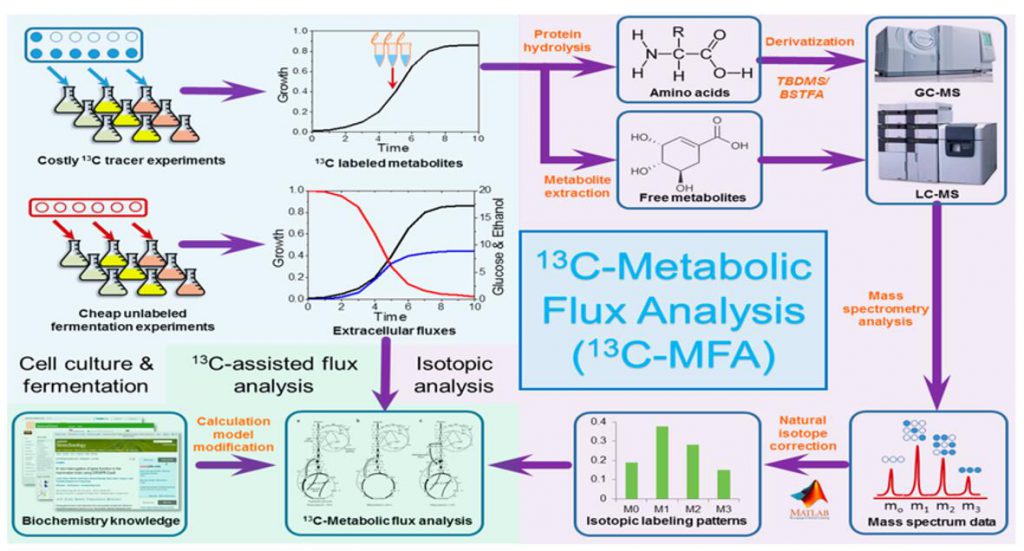
Metabolic flow analysis is not limited to specific metabolic pathways and can help us to solve the following problems.
(i) Isotope tracking of dynamically changing metabolite abundance information to uncover real metabolic flux information that is masked by traditional static metabolomics.
(ii) Mapping the recommended annotated isotopic metabolites into the KEGG pathway map to detect the full transmission of isotopic bodies in the organism without bias.
(iii) By integrating the interactions among multiple metabolic networks, the turnover rate, direction and distribution pattern of the compound in the intracellular metabolic pathway are deduced, and the relative contribution of multiple metabolic pathways to the overall cellular metabolism and their mutual regulation patterns are distinguished.
(iv) Mapping the recommended annotated metabolites into the KEGG reaction network to deeply mine the metabolic reactions and discover new functional information not identified by existing metabolic pathways.
Reference
Antoniewicz M R. Methods and advances in metabolic flux analysis: a mini-review. Journal of industrial microbiology & biotechnology, 2015, 42(3): 317-325.