Over 100 new biopharmaceuticals have been approved for the past decade, most of these newly marketed biopharmaceuticals utilizing recombinant proteins expressed by eukaryotic expression systems, and most of these recombinant proteins are glycoproteins such as monoclonal antibodies, fusion proteins, growth factors and the like. Since glycosylation will affect the safety and efficacy of glycoprotein drugs, the detailed characterization of glycosylation is essential for glycoprotein drug quality analysis and control, meanwhile, drug regulatory agencies have strict requirements for the stability of glycosylation analysis. Therefore, it has been a challenge for drug developers to establish an automated and reliable glycosylation analysis process.
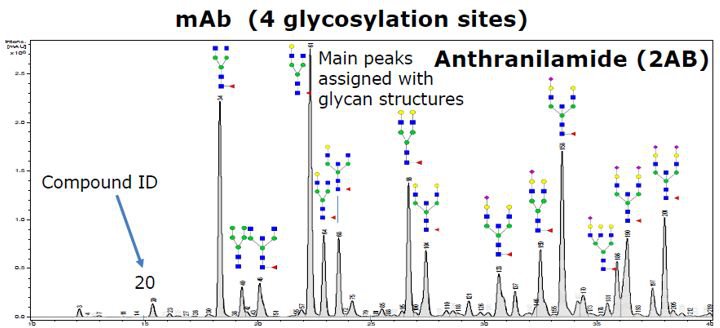
Fluorescent labeling based quantitative method, as the gold standard for quantitative analysis of Released Glycans (free glycoside), has been widely used in the quality control of monoclonal antibody drug process development and quality control of the final product. However, this retention time-based analysis method shows the disadvantage of insufficient specificity for some samples with more complex glycosylation. With regard to this, the exact mass of the glycoside is determined as a supplement to increase the specificity of the assay, but it is still not enough to make a 100% positive annotation of all glycosides in a complex sample.
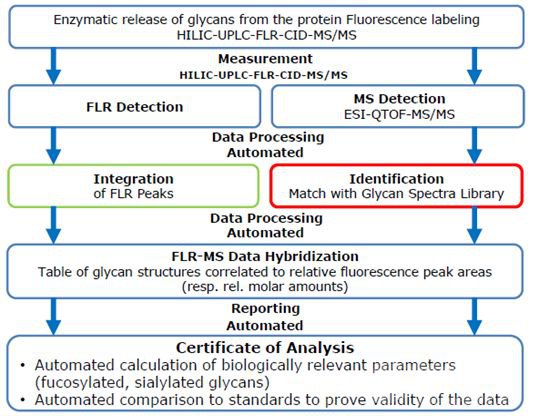
In response to this challenge, Glycotope and Brooke have collaborated to develop a workflow specifically for Released Glycans analysis, which combines the data obtained by Bruker's high-resolution QTOF with the fluorescence chromatogram produced by UPLC via GlycoFiler software, and providing an automated and reliable glycosylation qualitative and quantitative analysis process.
Highly automated data processing and report generation
After the Released Glycans sample is analyzed by UPLC-FLR-QTOF and the data acquisition is completed, GLycoFiler can automatically integrate the fluorescence chromatogram to obtain the peak area of each peak, and automatically search the MS/MS data to obtain the structural information of the glycoside. The entire search process can be completed in only 5 minutes, and then the glycoside structure is correlated with the corresponding fluorescent peak, which completes the qualitative and relative quantitative analysis of the glycoside, the automated reporting of this workflow is also very powerful. In addition to reporting the final structural identification and quantitative results, it can also be statistically based on the type of glycosylation (such as sialic acid, core fucose, high glycosides, etc.), as well as the comparative analysis between batches.
Glycoside structure identification and highly reliable data annotation
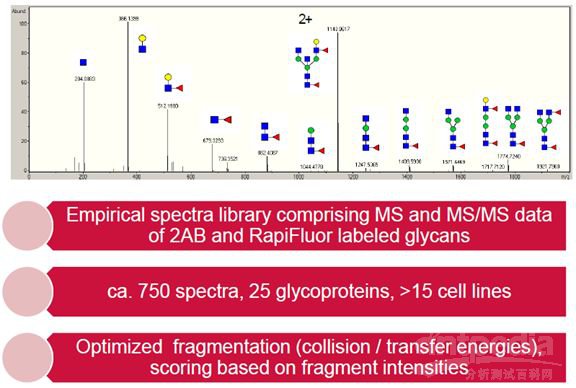
At present, glycosylation identification analysis is based on liquid-based retention time (such as GU value) and first-order accurate mass, which can be used for monoclonal antibody samples with relatively simple glycosylation, however, the reliability of identification results will be greatly reduced for the samples with more complex glycosylation (such as integrin, monoclonal antibody to glycosylation site, blood factor, etc.) And Bruker and GlycoTope jointly launched a glycosylation analysis program to search for glycosidic libraries to identify glycoside structures based on MS/MS data. The built-in glycoside library contains more than 750 glycoside experimental mass spectra (MS and MS/MS spectra of glycosides), which covers 25 glycoproteins in more than 15 cell lines and supporting 2-AB labeling and RapiFlour labeling.
Speed up the process of research and development of glycoprotein drugs
The Released Glycans analysis program can be widely applied in the early research of protein and ligand interaction in drug development, cloning screening, cell line development and fermentation process development, product characterization, product release test, and quality control in bioprocesses. The highly automated and highly reliable features of this assay will accelerate the speed of glycosylation analysis and the reliability of quality control, thus accelerating the development of glycoprotein drugs.