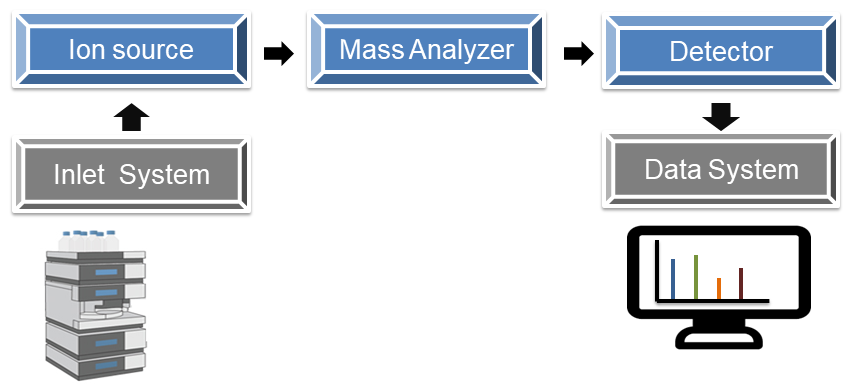
As we know before, the mass spectrometer consists of three main components, the ion source, the mass analyzer, and the detector. In ion source, a sample is ionized. Today, we are going to introduce several types of ion source which are usually used in a mass spectrometer.
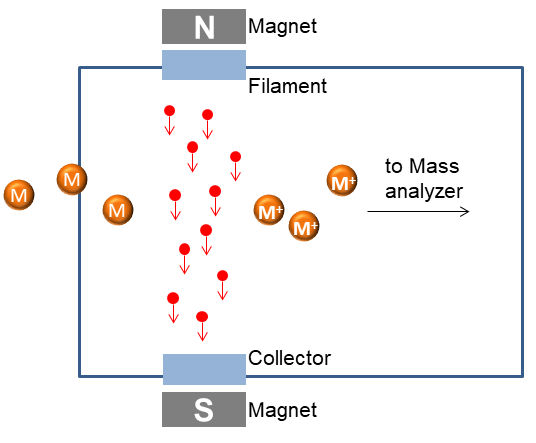
EI is one of the first and popular ionization methods for mass spectrometry. It is appropriate for organic molecules whose relatively molecule weight is below 600. In this technique, a current passes a wire filament can produce electrons for ionization. And an electric field accelerates these electrons to produce a beam of high energy electrons. When a molecule passes this high energy electrons beam, an electron can be expelled from the molecule to produce an ion.
EI works well for many gas phase molecules, so it always works with gas chromatography (GC), which can be incorporated for the separation of mixtures of thermally stable and volatile gases. Although EI works well for many gas phase molecules, it does have some disadvantages. EI causes extensive fragmentation, therefore the molecular ion is not observed for many compounds.
Chemical Ionization (CI)
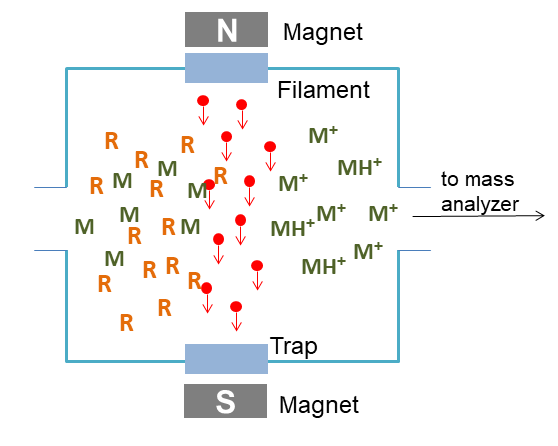
Chemical ionization is a lower energy process than EI. In CI, ions are produced by the collision of the samples with ions of a reagent gas that are present in the ion source. Inside the ion source, the reagent gas is extremely more than samples. Reagent gas is first subjected to electron impact to produce reagent gas ions by the fast electrons from the filament. Sample ions are produced by the ion-molecule reactions of reagent gas ions and sample molecules. In general, reagent gas molecules are present in the ratio of about 100:1 with respect to sample molecules. Some common reagent gases include methane, ammonia, and isobutene. Positive and negative ions of the sample are formed by reactions with this plasma. Like EI, CI usually works with gas chromatography. CI has the same general limitations as electron ionization mass spectrometry (EIMS) on the volatility and thermal stability of the compound being analyzed.
Atmospheric Pressure Chemical Ionization (APCI)
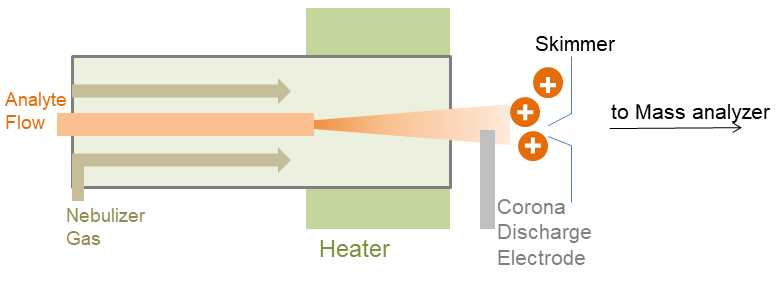
APCI is a method which creates ions at atmospheric pressure (105Pa), commonly coupled with high-performance liquid chromatography (HPLC). And this method is appropriate for relatively polar and semi-volatile samples. The APCI always consists of a nebulizer probe which can be heated to 350-500℃, an ionization region with a corona discharge needle and an ion-transfer region. A sample solution flows through a heated nebulizer where it is produced into a mist and the mist is transformed into a gas stream. When the gas stream arrived in the ionization region at atmospheric pressure, the gaseous solvent and sample are then ionized by a corona discharge, in which a highly charged electrode creates an electric field strong enough to ionize nearby molecules. The resulting sample ions then pass through a skimmer into the ion-transfer region. Ions are injected into the mass analyzer for detection.
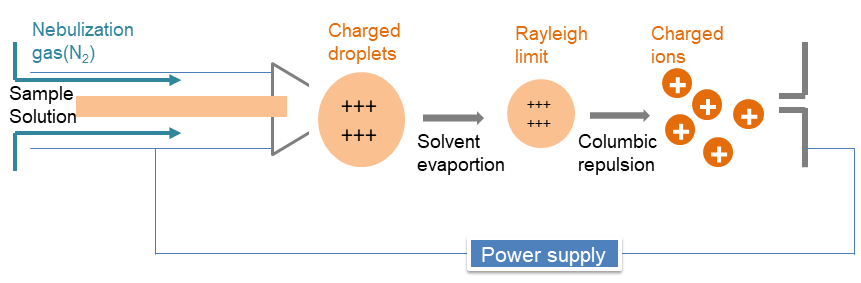
Electrospray ionization (ESI)
ESI is a soft ionization technique, which is useful for biological molecules of large molecular mass, because this process turns the macromolecule ionized into small droplets instead of fragmenting the macromolecules into smaller charged particles. ESI uses an electrical stress between the ESI probe exit and the counter electrode, which is located few millimeters from the probe. The process results in the generation of highly charged droplets directly from the infused solution. With the help of another stream of heat or dry gas, which are often called desolvation gas, the charged droplets are continuously reduced in size by evaporation of the solvent, leading to an increase of surface charge density and a decrease of the droplet radius. And it leads to the Columbic repulsion between the charges present in the droplet and then forms individual gas phase analyte ions (that critical point known as the Rayleigh limit). Finally, the ions are guided into a mass analyzer.
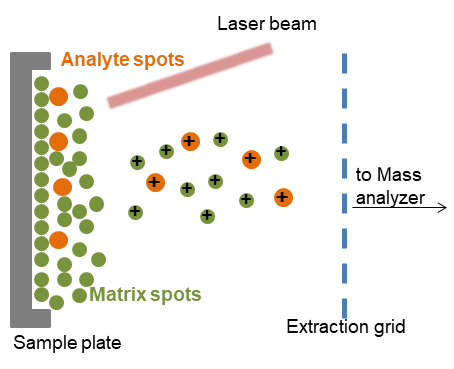
Matrix-Assisted Laser Desorption/Ionization (MALDI)
Matrix-assisted laser desorption/ionization (MALDI) is a technique to allow the high molecular weight compounds into the gas phase as intact ions. The mechanism of MALDI consists of three processes. Firstly, the sample is mixed with a suitable matrix material in excess and applied them to a plate. Secondly, a pulsed laser irradiates the sample, the matrix material cause rapid vibrational excitation by absorbing the laser irradiation, which leads to the localized disintegration of the solid solution. Finally, the analyte molecules are ionized by being protonated or deprotonated, and can then be accelerated into a mass analyzer. The time-of-flight (TOF) analyzers are always used with the MALDI ionization source.
References
1.Munson B. Chemical ionization mass spectrometry: theory and applications. Encyclopedia of Analytical Chemistry, 2006.
2.Ho C S, Lam C W K, Chan M H M, et al. Electrospray ionisation mass spectrometry: principles and clinical applications. The Clinical Biochemist Reviews, 2003, 24(1): 3.