Structural Diversity of Leukotrienes
Leukotrienes, derived from arachidonic acid metabolism, exhibit diverse structures, driving their distinct functions. Arachidonic acid serves as their common backbone. Leukotriene A4 (LTA4), a pivotal intermediate, undergoes enzymatic transformations to yield leukotriene B4 (LTB4) and cysteinyl leukotrienes (LTC4, LTD4, LTE4).
LTB4, characterized by a diene structure, acts as a potent chemoattractant, guiding immune cells to inflammation sites. Cysteinyl leukotrienes, bearing a thiol group, play roles in bronchoconstriction and inflammation. LTC4's peptide chain influences its binding to cell membranes. LTD4 induces bronchoconstriction and increased vascular permeability. LTE4, a stable metabolite, sustains cellular responses.
Structural diversity aligns with functional specificity, with LTB4 driving inflammation and cysteinyl leukotrienes contributing to allergic and asthmatic responses. Leukotriene receptors on target cells ensure precise cellular modulation. This structural complexity underpins the multifaceted roles of leukotrienes in various physiological processes and diseases.
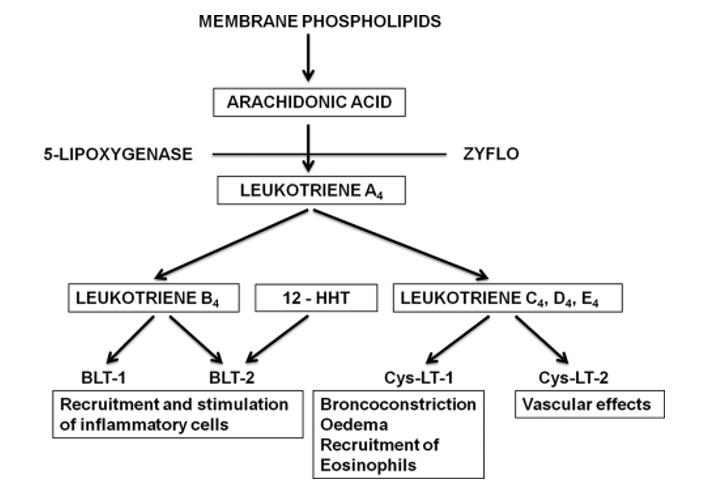
Formation and biological effects of leukotrienes (Samuelsson et al., 2012).
Leukotriene Modifiers and Leukotriene Inhibitors
Leukotriene modifiers are strategically categorized into two main groups - leukotriene inhibitors and leukotriene receptor antagonists. While both aim to modulate leukotriene activity, they do so through distinct mechanisms, targeting different points in the leukotriene biosynthetic and signaling pathways.
Leukotriene Inhibitors:
Leukotriene inhibitors, also known as 5-lipoxygenase inhibitors, operate at the initial stage of leukotriene biosynthesis. The pivotal enzyme in this process is 5-lipoxygenase, responsible for converting arachidonic acid into leukotriene A4. Notable among leukotriene inhibitors is Zileuton, which obstructs the enzymatic activity of 5-lipoxygenase, thereby curtailing the production of leukotrienes.
Mechanism of Action:
Zileuton, through its inhibition of 5-lipoxygenase, disrupts the formation of leukotriene A4. By doing so, it effectively attenuates the downstream synthesis of leukotrienes, preventing their engagement in inflammatory processes. This targeted approach makes leukotriene inhibitors particularly valuable in conditions where excessive leukotriene production contributes to pathology.
Leukotriene Receptor Antagonists:
Leukotriene receptor antagonists, such as montelukast and zafirlukast, function at a later stage in the leukotriene signaling pathway. Instead of inhibiting synthesis, these compounds selectively block the action of leukotrienes by binding to their receptors. Of particular interest are the receptors for cysteinyl leukotrienes, namely CysLT1 and CysLT2.
Mechanism of Action:
Montelukast, for instance, acts as a selective antagonist for the CysLT1 receptor. By binding to this receptor, montelukast hinders the downstream effects of cysteinyl leukotrienes, mitigating bronchoconstriction, inflammation, and mucus production. This targeted blockade proves beneficial in conditions where excessive leukotriene receptor activation contributes to pathological processes.
Physiological Functions of Leukotrienes
Leukotrienes, derived from the metabolism of arachidonic acid, serve as powerful lipid mediators with diverse physiological functions. These functions intricately contribute to the regulation of immune responses, inflammation, and the overall homeostasis of the body.
Immune Cell Recruitment
Leukotriene B4 (LTB4), a product of the 5-lipoxygenase pathway, acts as a potent chemoattractant. Its primary function lies in orchestrating the recruitment and activation of immune cells, especially neutrophils, to sites of infection or injury. LTB4 promotes the directional migration of immune cells, facilitating an effective immune response against pathogens.
Smooth Muscle Contraction and Bronchoconstriction
Cysteinyl leukotrienes, including LTC4, LTD4, and LTE4, exert profound effects on smooth muscle contraction. Their actions are particularly notable in the bronchioles, where they contribute to bronchoconstriction. In conditions such as asthma, the release of cysteinyl leukotrienes leads to airway narrowing, increased resistance, and respiratory symptoms.
Vascular Permeability
Cysteinyl leukotrienes also play a crucial role in regulating vascular permeability. By inducing the dilation of blood vessels and increasing their permeability, these mediators facilitate the movement of immune cells to sites of inflammation. This vascular response is integral to the overall inflammatory process, ensuring an adequate immune cell presence at the affected site.
Mucus Production
In addition to their effects on smooth muscle and vascular responses, cysteinyl leukotrienes contribute to mucus production. Increased mucus secretion in the airways is a protective mechanism aimed at trapping and expelling inhaled particles, pathogens, or irritants. In conditions like asthma, however, excessive mucus production can contribute to airway obstruction and respiratory distress.
Immune Modulation and Resolution
Leukotrienes play a vital role in modulating immune responses and resolving inflammation. While LTB4 promotes the initial recruitment of immune cells, other leukotrienes contribute to the subsequent resolution of inflammation. This dynamic interplay ensures that the immune system responds appropriately to challenges while preventing prolonged and excessive inflammation that could lead to tissue damage.
Analytical Methods for Leukotriene Analysis
Analyzing leukotrienes, crucial lipid mediators involved in inflammation and immune responses, requires sophisticated methods to understand their roles in health and disease. Various analytical techniques are employed to detect, quantify, and characterize leukotrienes, providing valuable insights into their physiological functions and dysregulation in pathological conditions.
High-Performance Liquid Chromatography (HPLC): Quantitative Precision
High-performance liquid chromatography (HPLC) is a widely utilized technique for the quantitative analysis of leukotrienes. HPLC separates complex mixtures based on their interactions with a stationary phase and a mobile phase. Detection methods, often coupled with mass spectrometry (MS) or ultraviolet (UV) detectors, allow for the precise quantification of individual leukotrienes. This method is valuable for studying the profile of leukotrienes in biological samples, providing quantitative data crucial for understanding their roles in specific physiological contexts.
Mass Spectrometry (MS): Unraveling Structural Details
Mass spectrometry is a powerful tool for the identification and structural elucidation of leukotrienes. Liquid chromatography-mass spectrometry (LC-MS) is commonly employed for its sensitivity and ability to distinguish between different leukotriene isomers. MS allows researchers to characterize the molecular weights and fragmentation patterns of leukotrienes, facilitating a comprehensive understanding of their structural diversity and modifications.
Enzyme-Linked Immunosorbent Assay (ELISA): Targeted Detection
Enzyme-linked immunosorbent assay (ELISA) is a targeted method used for the quantification of specific leukotrienes in biological samples. This immunological assay relies on the interaction between a leukotriene-specific antibody and the target molecule. ELISA is particularly valuable for screening and quantifying leukotrienes with high specificity, providing researchers with a focused approach to studying the involvement of specific leukotrienes in various biological processes.
Gas Chromatography-Mass Spectrometry (GC-MS): Separation and Identification
Gas chromatography-mass spectrometry (GC-MS) is employed for the separation and identification of volatile and semi-volatile compounds, including certain leukotrienes. This technique involves the vaporization of samples and their passage through a chromatographic column, followed by mass spectrometric analysis. GC-MS is advantageous for its ability to separate isomeric compounds, aiding in the precise identification of specific leukotriene species.
Nuclear Magnetic Resonance (NMR): Structural Insights
Nuclear magnetic resonance spectroscopy (NMR) is utilized for gaining structural insights into leukotrienes. By exploiting the magnetic properties of certain nuclei, NMR provides information about the spatial arrangement of atoms in a molecule. This technique aids in confirming the identity and elucidating the three-dimensional structures of leukotrienes, contributing valuable information to complement other analytical methods.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS): Sensitivity and Specificity
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) combines the separation capabilities of liquid chromatography with the sensitivity and specificity of mass spectrometry. LC-MS/MS is a robust method for simultaneously quantifying multiple leukotrienes in complex biological matrices. This technique allows researchers to explore the dynamic changes in leukotriene profiles, offering a comprehensive view of their involvement in physiological processes.
Reference
Samuelsson, Bengt. "Role of basic science in the development of new medicines: examples from the eicosanoid field." Journal of Biological Chemistry 287.13 (2012): 10070-10080.