Host cell proteins (HCPs) are one of the key process-related impurities generated during the production of biologics, which can lead to adverse immune reactions and hypersensitivity reactions in patients. There are also problems that lead to the degradation of product proteins, which affects the safety and efficacy of the product and is a key quality control indicator.
HCPs Detection Method
HCPs residue detection is the most widely used method is ELISA, simple operation, high sensitivity, high throughput, is the daily release test method for HCPs residue detection. National pharmacopoeias provide for the use of enzyme immunoassay for HCPs residue detection.
However, ELISA methods for the detection of HCP residues still face many challenges. In the actual process of production, the host source, production process, purification method, production scale, etc. may have an impact on the type and complexity of HCPs. For example, the use of commercial general-purpose HCP ELISA kits for detecting HCPs in products is prone to missed detection due to their low antibody specificity and applicability, posing a risk to the quality and safety of biologics. In addition, commercial HCP ELISA kits need to be validated again when changing batches.
In the production of monoclonal antibody drugs, HCPs residues can be efficiently removed by Protein A. However, gene therapy drugs such as AAV and lysozyme virus do not have such a process to remove HCPs. However, there is no such process to remove HCPs in the production of gene therapy drugs, such as AAV and lysovirus, etc. Therefore, especially for CGT products, it is necessary to analyze and detect HCPs residues in an integrated way.
With the development of proteomics technology, 2D electrophoresis, LC-MS and other methods are used to assist in the analysis of the composition of HCPs in biological products, which can more objectively and comprehensively verify the specificity of HCP ELISA kit detection and the actual HCP coverage 2D-SDS-PAGE, an orthogonal method relative to ELISA, can be used for the characterization and analysis of HCPs.
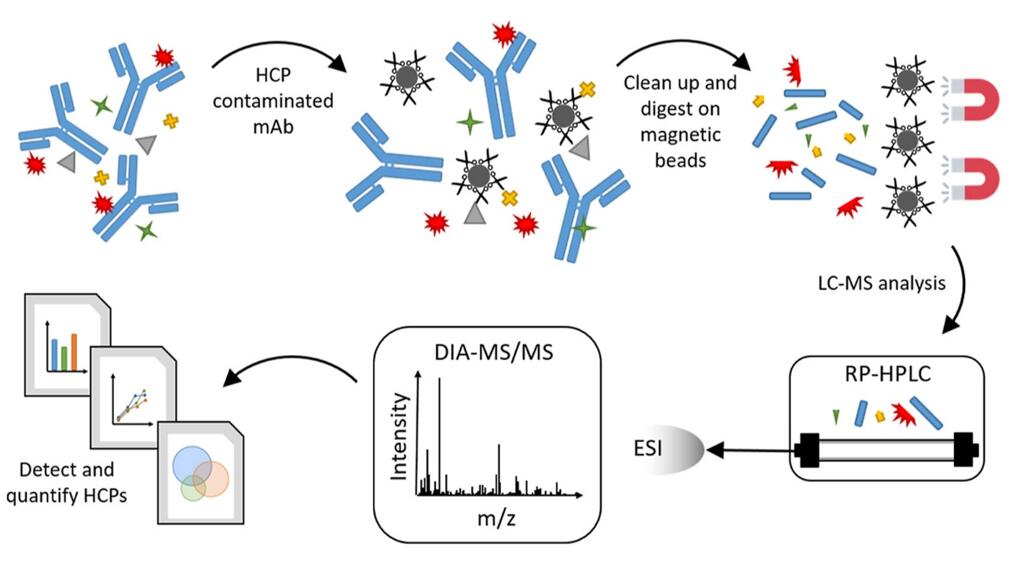
HCPs LC-MS/MS Analysis (Strasser et al., 2021)
HCPs Analysis Strategy
In view of the above risks and regulatory requirements, it is recommended that the following elements be considered when performing a full-scale assay analysis for HCPs.
- Pre-product development assays using ELISA must be validated for suitability, including analytical verification of specificity and antibody coverage; antibody coverage can be verified by orthogonal analysis using 2D electrophoresis and mass spectrometry methods.
- Auxiliary use of 2D, MS and other proteomics techniques to analyze the residues and composition of HCPs in the actual process samples, especially the specific high-risk HCPs that may be present in them, can effectively improve the accuracy of detection results and ensure product quality and safety.
Based on ELISA technology and Orbitrap mass spectrometry platform, Creative Proteomics can provide you with HCPs detection services for HCPs residue identification in biologics, HCPs antibody coverage analysis, etc.
Reference
Strasser, L., Oliviero, G., et al. (2021). Detection and quantitation of host cell proteins in monoclonal antibody drug products using automated sample preparation and data-independent acquisition LC-MS/MS. Journal of Pharmaceutical Analysis, 11(6), 726-731.