Recently, the high performance thin layer chromatography---HPTLC, as a rapid and simple method, is used to determine DPH and NPS from pharmaceutical preparation simultaneously. Diphenhydramine hydrochloride is an antihistamines, which appear to compete with histamine for cell receptor sites on effector cell, with anticholinergic and seda-tive effects. And Naproxen sodium is 2-propanoate sodium.
Materials
Chemicals and reagents
Purifying DPH and NPS and receiving as a gift sample from Souvin Pharmaceuticals and Rubicon Pharmaceuticals, respectively. And then combining and formulating 25mg of DPH and 200mg of NPS. The solvents, including toluene, methanol and glacial acetic acid, should all be of AR grade.
Instrumentation
Microsyrine, which should be precoated with silica gel 60 F254 glass plates, Camag 10 μL sample syringe, Camag Linomat V automatic sample applicator, Camag twin through chamber 10 x 10 cm, TLC scanner III, UV chamber and win CATS version 1.4.0 sofe-ware all will be applied in this experiment.
Conditions of chromatography
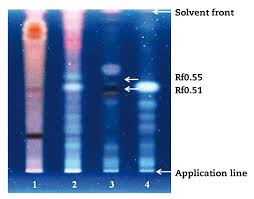
5 μL of standard solution and 5 μL sample solution are utilized on the TLC plate by applying Camag Linomat V automatic sample applicator in the form of band by using microsyringe. Using 150 nLs of a constant application rate. Using a twin through glass chamber , which should be with the mobile phase of toluene, to saturate the plates for 20 minutes. And then, the plates should be placed in the mobile phase, performing ascend-ing development to a distance of 8cm. And the plates were air dried, performing densitomertric scanning at 230nm by using Camag TLC scanner III, which should be operated in reflectance-absorbance mode.
Method
Accuracy: the weighed quality of a sample should be equivalent to around 10 mg DPH and 88 mg NPS, which then should be transferred in nine 25.0 mL volumetric flasks, adding 8/70.4 mg, 10/88 mg, and 12/105.6 mg of DPH/NPS to the sample for 80%, 100%, and 120% level of recovery. Performing all dilutions with methanol. Preparing and analyzing solutions in triplicate.
Precision
Performing precision researches to guarantee the repeatability and reproducibility of the method. Preparing and analyzing the sample solution in the similar manner mentioned above. Analyzing a sample solution at three different time intervals to make sure the intraday precision, which then should be determined by analyzing a sample solution on three consecutive days.
Robustness
Small but deliberate changes in the optimized method parameters were done to evaluate the robustness of the proposed method. By introducing small changes in the mobile phase composition, mobile phase volume, duration of chamber saturation with mobile phase allows researchers to determine the time from spotting, time from development, the effects on Rf value and peak area of drugs.
Detection and quantitation limitations
Using 3a/S and 10a /S phe-nomena for the limits of detection and quantification, respec-tively to calculate the LOD and LOQ.
Forced degradation studies
Exposing a sample to stress conditions, including acidic, alkaline and oxidation to try the intentional degradation, in which the contents of flasks were refluxed in a three-hour water bath at 80℃. As for the heat and photo degradation, a sample was kept at 60℃ and in UV light for one day. Removing and allowing flasks to cool after the respective time intervals. And then preparing and analyzing samples in the similar way.
HPTLC is now widely applied in many biological aspects.
Related service: