Protein lipidation is a reversible post-translational modification in which lipid groups are covalently attached to specific amino acid residues of target proteins. This process alters the protein's hydrophobicity, subcellular localization, stability, and functional interactions. Lipidation is a crucial regulator of protein function, particularly in membrane association and signaling dynamics.
By adding fatty acids, isoprenoids, or cholesterol-like molecules, the cell gives the protein a kind of "anchor" that helps it stick to membranes. This allows the protein to reach the correct location, especially on or near cell membranes, and participate in essential tasks like sending signals, moving materials, or guiding immune responses. Lipidation is necessary for healthy cell activity because of its role in controlling protein placement and function.
Types of Protein Lipidation Modifications
Protein lipidation encompasses several structurally distinct modifications. Each type involves specific enzymatic machinery and targets defined protein motifs. These modifications include N-myristoylation, S-palmitoylation, prenylation, and other less common lipid attachments.
N-Myristoylation
N-myristoylation is a permanent modification that happens right after a protein is made. A 14-carbon saturated fatty acid called myristic acid is attached to the protein's first amino acid—usually glycine—after removing the starting methionine. This reaction is carried out by an enzyme called N-myristoyltransferase (NMT). Once this lipid is added, the protein can loosely associate with membranes inside the cell.
- Why it matters: N-myristoylation is involved in many essential cellular activities, including cell signaling, immune responses, and programmed cell death. Several viruses also use this mechanism to ensure their proteins can reach the right parts of host cells.
- Example: Proteins like the Src family kinases and HIV Gag are myristoylated to help them reach the plasma membrane, where they perform their functions.
S-Palmitoylation
S-palmitoylation is the attachment of a 16-carbon fatty acid—palmitic acid—to cysteine residues on proteins. This bond is reversible, meaning the lipid can be added and later removed. This flexibility allows the cell to regulate where the protein goes and when it is active. The enzymes that add the lipid are called DHHC palmitoyltransferases, named after their conserved amino acid motif. Specialized enzymes called palmitoyl-protein thioesterases remove the lipid when it is no longer needed.
- Why it matters: S-palmitoylation is like a "GPS signal" that directs proteins to membranes, especially in the brain and immune system. It plays a big role in processes like learning, memory, and inflammation.
- Example: The Ras family of proteins, which help control cell growth, need to be palmitoylated to work properly. Without this modification, Ras stays in the wrong part of the cell and cannot transmit growth signals.
Prenylation (Farnesylation & Geranylgeranylation)
Prenylation involves the addition of long carbon chains called isoprenoids to proteins. These can be farnesyl (15-carbon) or geranylgeranyl (20-carbon) chains. The process usually targets a sequence at the end of the protein known as the CAAX box, where "C" is cysteine and "X" determines which type of lipid is attached. Two main enzymes do the work: farnesyltransferase (FTase) and geranylgeranyltransferase (GGTase)
- Why it matters: Prenylation is especially important for proteins that regulate cell shape divis.
- Example: KRAS, a key protein in many cancers, must be farnesylated to send growth signals. Blocking this process with farnesyltransferase inhibitors (FTIs) is being explored as a potential cancer treatment.
Other Modifications
Additional lipidation forms include glycosylphosphatidylinositol (GPI) anchoring and cholesterol modification. GPI anchoring is a post-translational modification that targets proteins to the outer leaflet of the plasma membrane, especially within lipid rafts. Cholesterol modification, particularly relevant in Hedgehog signaling, involves the covalent addition of cholesterol to the C-terminal end of signaling proteins.
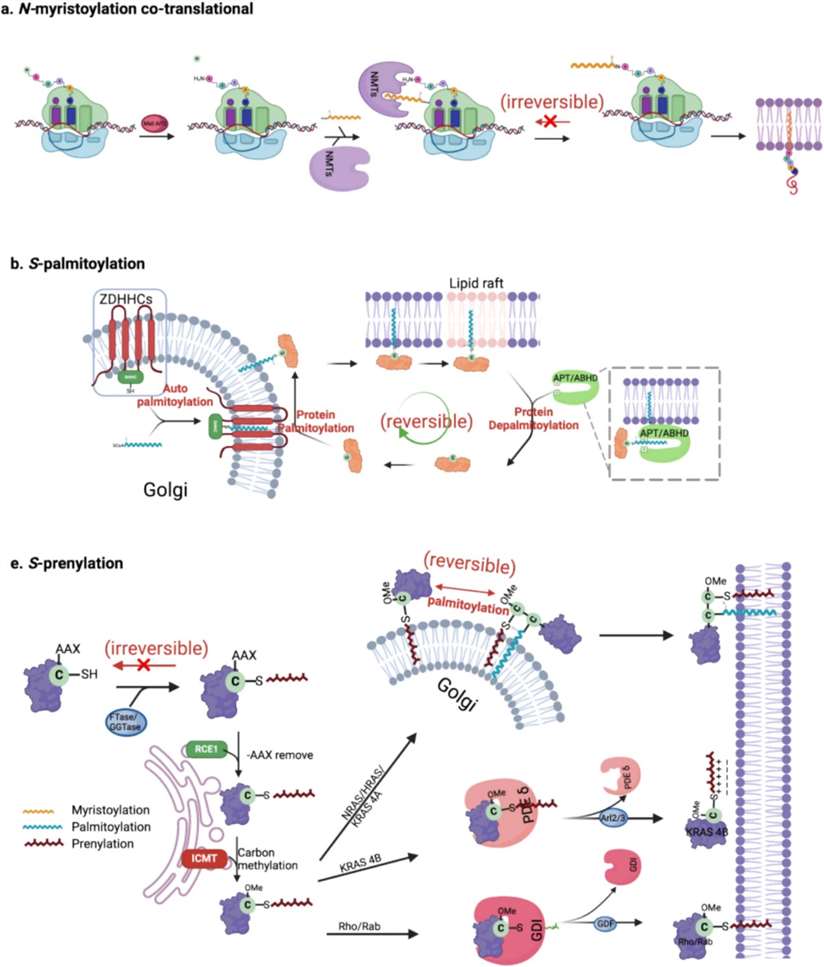 Figure 1. Processes of the three major classes for protein lipidation. (Xu M, et al., 2025)
Figure 1. Processes of the three major classes for protein lipidation. (Xu M, et al., 2025)
Analysis and Identification of Protein Lipidation
| Lipidation Type | Main Analytical Strategy | Labeling Method | Enrichment Technique | MS Detection Method | Applications |
|---|---|---|---|---|---|
| N-myristoylation | Metabolic labeling with fatty acid analogs | Azido-/alkyne-myristic acid analogs (e.g., tetradec-13-ynoic acid) | Click chemistry + avidin-biotin purification | LC-MS/MS; enhanced by cleavable linkers | Can suffer from off-target labeling; refined using NMT inhibitors and N-terminal glycine profiling. Widely used in parasitic research. |
| S-palmitoylation | Metabolic labeling or Acyl-Biotin Exchange (ABE) | Azido-/alkyne-palmitic acid analogs; Native cysteine thioester chemistry | Click chemistry; Hydroxylamine cleavage + thiol-reactive biotin | LC-MS/MS | ABE allows site-specific capture of native palmitoylated cysteines; labeling methods are compatible with dynamic palmitoylation studies. |
| Prenylation | Bioorthogonal chemical labeling | Azido/alkyne-farnesyl or geranylgeranyl analogs | Staudinger ligation, CuAAC, affinity tags (biotin) | LC-MS/MS (MudPIT, CID, ECD, ETD) | Specific for FTase or GGTase; can be adapted to study enzyme specificity and pathogen prenylomes. |
Mechanisms Linking Lipidation to Cell Signaling Pathways
Protein lipidation governs critical aspects of signal transduction through multiple mechanisms. It affects membrane association, spatial compartmentalization, protein-protein interactions, and conformational transitions.
Lipid Modifications and Protein-Membrane Interactions
Lipidation helps proteins stick to cellular membranes, which are the gatekeepers of biological signaling. Many signaling proteins only become active after they move to the inner side of the cell membrane. Lipid tags act like anchors, drawing these proteins to specific membrane regions.
For instance, myristoylation—the attachment of a 14-carbon fatty acid called myristate—provides a weak but persistent signal that guides proteins to membranes. Palmitoylation, which adds a longer fatty acid called palmitate, strengthens this attachment. Proteins like Src family kinases or G proteins rely on these lipid anchors to reach the membrane and activate downstream targets involved in cell growth, metabolism, and movement.
Without proper lipidation, these proteins can float freely in the cell's watery interior, unable to interact with the membrane or initiate signaling.
Role in Membrane Microdomain Localization
Cell membranes are not uniform. They contain specialized zones called lipid rafts, which are rich in cholesterol and sphingolipids. These microdomains act like "control centers" that gather key signaling molecules into close proximity, allowing them to interact efficiently.
Lipidation often determines whether a protein can enter or stay within these zones. For example, palmitoylated proteins, such as Ras and Lyn kinase, are more likely to be found in lipid rafts. Once localized in these hubs, they can quickly turn on or off critical signaling pathways, including those that regulate immune responses, inflammation, or cell survival.
By steering proteins into specific membrane zones, lipidation helps organize and streamline signaling—much like assigning team members to the right part of a control room
Impact on Protein-Protein Interactions and Conformational Dynamics
Besides steering proteins to the right spot, adding lipid tags can alter their shape and how they stick to partners. That, in turn, changes how well they team up with other proteins and send messages inside the cell.
For instance, prenylation—a type of lipidation involving farnesyl or geranylgeranyl groups—enables proteins like Ras and Rho GTPases to adopt active shapes at the membrane. Once activated, these proteins interact with effectors that control cell shape, movement, and survival.
Moreover, reversible lipid modifications such as palmitoylation can act as molecular switches. Proteins can be palmitoylated or depalmitoylated in response to cellular signals, causing them to cycle between active (membrane-bound) and inactive (cytoplasmic) states. This dynamic behavior adds another layer of control, allowing cells to fine-tune their responses based on environmental cues.
Protein Lipidation in Disease Pathology
Oncogenic Signaling: Lipidation of RAS and Beyond
Aberrant protein lipidation plays a pivotal role in the pathogenesis of numerous human diseases. In cancer, the hyperactivation of lipid-modified small GTPases, particularly RAS family proteins, is one of the most studied oncogenic mechanisms. Farnesylation and geranylgeranylation facilitate the membrane localization of RAS isoforms, which is essential for their signaling activity. Mutations in KRAS and HRAS, commonly observed in pancreatic, lung, and colorectal cancers, depend on proper prenylation for their oncogenic function (Cox et al., 2015; Ahearn et al., 2018). Disruption of these lipid anchors can reduce proliferative signaling via the MAPK and PI3K pathways.
Neurodegenerative Disorders: Palmitoylation Defects
In neurodegenerative disorders, protein palmitoylation defects have been associated with synaptic dysfunction and neuronal toxicity. Huntingtin (HTT), the mutated protein in Huntington's disease, undergoes abnormal palmitoylation, which influences its aggregation and cytotoxicity (Yanai et al., 2006). Similarly, misregulation of PSD-95 palmitoylation impairs synaptic targeting, contributing to cognitive decline in Alzheimer's disease (El-Husseini et al., 2002; Fukata and Fukata, 2010).
Infectious Diseases: Pathogen-Mediated Lipidation Manipulation
In infectious diseases, several pathogens manipulate host lipidation machinery to facilitate infection and immune evasion. For instance, Mycobacterium tuberculosis secretes effectors that hijack host palmitoylation enzymes to reprogram macrophage function (Tundup et al., 2014). Moreover, Plasmodium falciparum, the causative agent of malaria, relies on N-myristoylation of key proteins for parasitic growth and survival in erythrocytes (Wright et al., 2014).
Therapeutic Targeting of Protein Lipidation
Given its central role in disease, protein lipidation has emerged as a promising therapeutic target. Several strategies are under investigation, including small-molecule inhibitors, biologics, and lipid-based drug delivery systems.
Small-Molecule Inhibitors of Lipidation Enzymes
Therapeutic targeting of protein lipidation has gained considerable traction as a novel strategy to modulate aberrant signaling in various diseases. Small-molecule inhibitors directed at lipidation enzymes, such as farnesyltransferase (FTase) and geranylgeranyltransferase (GGTase), have been extensively studied. Tipifarnib, a selective FTase inhibitor, has advanced into clinical trials, demonstrating efficacy particularly in HRAS-mutant cancers (Ahearn et al., 2018; Cox et al., 2015). Beyond farnesylation, inhibitors of N-myristoyltransferase (NMT) have shown promising antiproliferative effects in hematological malignancies and parasitic infections (Thinon et al., 2016; Wright et al., 2014).
Palmitoylation inhibitors such as 2-bromopalmitate (2-BP) have been widely used experimentally to disrupt the dynamic attachment of palmitic acid, although its lack of specificity limits clinical translation (Webb et al., 2000). More recently, efforts to develop selective inhibitors of palmitoyl acyltransferases (PATs) have begun, highlighting the therapeutic potential in neurodegenerative disorders and cancer (Greaves and Chamberlain, 2011).
Emerging Biologics & Peptidomimetics
Emerging biologics and peptidomimetics provide alternative approaches by specifically interfering with lipid-protein interactions. For example, peptidomimetic molecules that mimic lipidation recognition motifs competitively inhibit enzyme-substrate binding with enhanced selectivity (Resh, 2013). Additionally, antibody-based agents designed to recognize lipidated epitopes may block aberrant membrane localization in oncogenic signaling (Zhang et al., 2019).
Drug Delivery Strategies Leveraging Lipidation
Lipidation also facilitates advanced drug delivery systems. Lipidated peptides and proteins exhibit improved membrane permeability and serum stability, optimizing therapeutic index (Lundberg et al., 2020). Nanoparticles and liposomes engineered with lipid moieties exploit natural membrane targeting mechanisms to deliver nucleic acids and small molecules efficiently (Cullis and Hope, 2017). This strategy underpins recent successes in mRNA vaccine technology and targeted cancer therapies (Hou et al., 2021).
References
- Triola G. The protein lipidation and its analysis. Journal of Glycomics and Lipidomics, 2011, 2: 2153-0637. DOI: 10.4172/2153-0637.S2-001
- Xu M, Xu B. Protein lipidation in the tumor microenvironment: enzymology, signaling pathways, and therapeutics. Molecular Cancer, 2025, 24(1): 1-50. DOI: 10.1186/s12943-025-02309-7
- Triola, G. Protein Lipidation, Elucidation by Chemical Proteomics, and Its Functional Roles. In: Geiger, O. (eds) Biogenesis of Fatty Acids, Lipids and Membranes. Handbook of Hydrocarbon and Lipid Microbiology . Springer, Cham. 2018. DOI: 10.1007/978-3-319-43676-0_50-1
Our products and services are for research use only.